
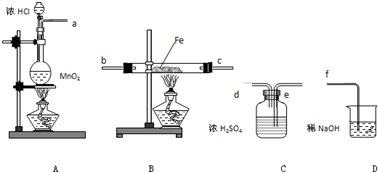
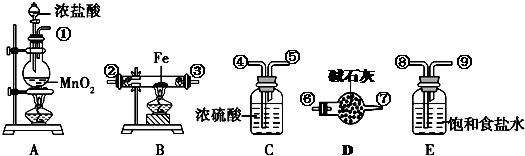
£Ø18·Ö£©£Ø1£©ŌŚŹµŃéŹŅÖĘČ”ÕōĮóĖ®µÄ×°ÖĆÖŠ£¬ĪĀ¶Č¼ĘµÄĖ®ŅųĒņÓ¦Ī»ÓŚ £»ÉÕĘæÖŠÓ¦·Å¼øĮ£·ŠŹÆ£Ø»ņĖé“Éʬ£©£¬Ęä×÷ÓĆŹĒ £»ĄäÄż¹ÜÖŠĄäÄżĖ®µÄĮ÷ĻņÓ¦µ±ŹĒ £ØĮ÷ĻņÓĆÉĻæŚ”¢ĻĀæŚ”¢ĖĪŖ½ųĖ®æŚ£¬ĖĪŖ³öĖ®æŚ±ķŹ¾£©”£
£Ø2£©ŹµŃéŹŅÓūÅäÖĘ0.5 mol”¤L-1µÄNaOHČÜŅŗ500 mL£¬ÓŠŅŌĻĀŅĒĘ÷£ŗ
¢ŁÉÕ± ¢Ś100 mLĮæĶ² ¢Ū1000 mL ČŻĮæĘæ ¢Ü500 mL ČŻĮæĘæ ¢Ż²£Į§°ō ¢ŽĶŠÅĢĢģĘ½£Ø“ųķĄĀė£©
a£ŗÅäÖĘŹ±£¬±ŲŠėŹ¹ÓƵÄŅĒĘ÷ÓŠ””””””””””£ØĢī“śŗÅ£©£¬»¹Č±ÉŁµÄŅĒĘ÷ŹĒ””””””””£¬øĆŹµŃéÖŠĮ½“ĪÓƵ½²£Į§°ō£¬Ęä×÷ÓĆ·Ö±šŹĒ”””””””””””””””¢”””””””””””””””””””£
b£ŗÅäÖĘŹ±£¬Ņ»°ćæÉ·ÖĪŖŅŌĻĀ¼øøö²½Öč£ŗ¢Ł³ĘĮæ ¢Ś¼ĘĖć ¢ŪČܽā ¢ÜŅ”ŌČ ¢Ż×ŖŅĘ ¢ŽĻ“µÓ ¢ß¶ØČŻ ¢ąĄäČ“£¬ĘäÕżČ·µÄ²Ł×÷Ė³ŠņŹĒ ”£
c£ŗŌŚÅäÖĘNaOHČÜŅŗŹ±£¬ĻĀĮŠ²Ł×÷»įµ¼ÖĀ½į¹ūĘ«µĶµÄŹĒ
£Ø1£©ÓĆĶĻÅĢĢģĘ½³ĘĮæŹ±ķĄĀė·ÅŌŚ×óÅĢ£Ø2£©ČܽāNaOH¹ĢĢåŗóƻӊĄäČ“ÖĮŹŅĪĀ¾ĶĻņČŻĮæĘæ×ŖŅĘČÜŅŗ
£Ø3£©½«ÉÕ±ÖŠµÄČÜŅŗ×ŖŅʵ½ČŻĮæĘæŹ±²»É÷Č÷µ½ČŻĮæĘæĶā£Ø4£©¶ØČŻŹ±ø©ŹÓæĢ¶ČĻߣØ5£©¶ØČŻŹ±ŃöŹÓæĢ¶ČĻß
£Ø6£©øɾ»µÄČŻĮæĘæĪ“¾øÉŌļ¾ĶÓĆÓŚÅäÖĘČÜŅŗ
 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā


| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com