
��1�����ԣ�H2CO3________H2SiO3��H2SiO3________H3PO4
��2�����ԣ�Ca(OH)2________Mg(OH)2��Mg(OH)2________Al(OH)3
��3����̬�⻯���ȶ��ԣ�H2O________H2S��H2S________HCl
��4����ԭ�ԣ�H2O________H2S��H2S________HCl
��5�����ԣ�H2SO4________H2SO3��HClO4________HClO
�����ϴ��п��Թ��ɳ���
��Ԫ�صķǽ�����Խǿ�����Ӧ���������ˮ���������Խ________��
��Ԫ�صĽ�����Խǿ�����Ӧ���������ˮ����ļ���Խ________��
��Ԫ�ص�________��Խǿ�����Ӧ��̬�⻯����ȶ���Խ________��
�ܷǽ�����Խǿ��Ԫ�����ɵ���̬�⻯��仹ԭ��Խ________��
��ͬ�ַǽ���Ԫ���γɵĺ����ᣬ�����Ԫ�ؼ�̬Խ�ߣ�������ҲԽ________��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013������ʡʵ����ѧ��У����12���¿���ѧ�Ծ����������� ���ͣ������
(10��) ���ݻ�Ϊ2L���ܱ������м���2 mol A��0.6 mol C��һ������B�������塣һ�������·�����Ӧ��������Ũ����ʱ��仯��ͼ1��ʾ��ͼ2Ϊt2ʱ�̺�ı䷴Ӧ������ƽ����ϵ�з�Ӧ������ʱ��仯����������ĸ��ζ����ı�һ�ֲ�ͬ��������
��֪t3��t4��Ϊʹ�ô�����ͼ1��t0��t1��c(B)δ������
(1) ��t1��15 min����t0��t1����CŨ�ȱ仯��ʾ�ķ�Ӧ����Ϊv(C)�� ��
(2) t4��t5�θı������Ϊ ��B����ʼ���ʵ���Ϊ ������ƽ��ʱ��Ӧ��ƽ�ⳣ�����±���ʾ��
| t1��t2 | t2��t3 | t3��t4 | t4��t5 | t5��t6 |
| K1 | K2 | K3 | K4 | K5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�����ʡ�����е�һ��ѧ�߶�1���¿���ѧ�Ծ� ���ͣ������
(1) ��Ӧ: 2A(g) B(g ) xC(g) ��һ���������´ﵽƽ��״̬, �ܱ������е�ѹǿ����P%, ���A��ת����Ϊ P%, ��x��ֵΪ __________.
B(g ) xC(g) ��һ���������´ﵽƽ��״̬, �ܱ������е�ѹǿ����P%, ���A��ת����Ϊ P%, ��x��ֵΪ __________.
(2) ���ݻ���ͬ�����ܱ�����A��B��, �����¶�Ϊ 423K, ͬʱ��A��B�зֱ���� a mol �� b mol ��HI(a > b), ����Ӧ: 2HI(g) H2(g)+ I2(g)��ƽ���, �� �� > < �� ="��" �ش���������:
H2(g)+ I2(g)��ƽ���, �� �� > < �� ="��" �ش���������:
�ӷ�Ӧ��ʼ���ﵽƽ������Ҫʱ��: tA _______ tB����>��<�� =", " ��ͬ��
ƽ��ʱ�ĵ��Ũ��: c(I2 )A c(I2 )B
HI��ƽ��ֽ�ٷ���Ϊ:  .
.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�������ʡ˫Ѽɽ�е�һ��ѧ�����������¿���ѧ�Ծ����������� ���ͣ������
(9��) ��һ���������ܱ������м���2 mol A��0.6 mol C��һ������B�������塣
һ�������·�����Ӧ��������Ũ����ʱ��仯��ͼ1��ʾ��ͼ2Ϊt2ʱ�̺�ı䷴Ӧ������ƽ����ϵ�з�Ӧ������ʱ��仯����������ĸ��ζ����ı�һ�ֲ�ͬ��������
��֪t3��t4��Ϊʹ�ô�����ͼ1��t0��t1��c(B)δ������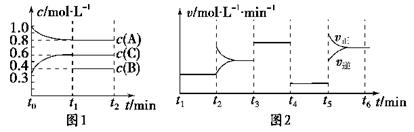
(1)��t1��15 min����t0��t1����CŨ�ȱ仯��ʾ�ķ�Ӧ����Ϊv(C)��________��
(2)t4��t5�θı������Ϊ________��B����ʼ���ʵ���Ϊ________������ƽ��ʱ��Ӧ��ƽ�ⳣ�����±���ʾ��
| t1��t2 | t2��t3 | t3��t4 | t4��t5 | t5��t6 |
| K1 | K2 | K3 | K4 | K5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ��У����12���¿���ѧ�Ծ��������棩 ���ͣ������
(10��) ���ݻ�Ϊ2L���ܱ������м���2 mol A��0.6 mol C��һ������B�������塣һ�������·�����Ӧ��������Ũ����ʱ��仯��ͼ1��ʾ��ͼ2Ϊt2ʱ�̺�ı䷴Ӧ������ƽ����ϵ�з�Ӧ������ʱ��仯����������ĸ��ζ����ı�һ�ֲ�ͬ��������
��֪t3��t4��Ϊʹ�ô�����ͼ1��t0��t1��c(B)δ������

(1) ��t1��15 min����t0��t1����CŨ�ȱ仯��ʾ�ķ�Ӧ����Ϊv(C)�� ��
(2) t4��t5�θı������Ϊ ��B����ʼ���ʵ���Ϊ ������ƽ��ʱ��Ӧ��ƽ�ⳣ�����±���ʾ��
|
t1��t2 |
t2��t3 |
t3��t4 |
t4��t5 |
t5��t6 |
|
K1 |
K2 |
K3 |
K4 |
K5 |
��K1��K2��K3��K4��K5֮��Ĺ�ϵΪ (�á�>����<����������)��
(3) ����ͬ�����£�����ʼʱ�����м���a mol A��b mol B��c mol C���ﵽƽ��ʱ��ϵ�и����ʵ�����t1ʱ����ȣ�a��b��cҪ���������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�����ʡ�����и߶�1���¿���ѧ�Ծ� ���ͣ������
(1) ��Ӧ: 2A(g) B(g ) xC(g) ��һ���������´ﵽƽ��״̬, �ܱ������е�ѹǿ����P%, ���A��ת����Ϊ P%, ��x ��ֵΪ __________.
B(g ) xC(g) ��һ���������´ﵽƽ��״̬, �ܱ������е�ѹǿ����P%, ���A��ת����Ϊ P%, ��x ��ֵΪ __________.
(2) ���ݻ���ͬ�����ܱ�����A��B��, �����¶�Ϊ 423K, ͬʱ��A��B�зֱ���� a mol �� b mol ��HI(a
> b), ����Ӧ: 2HI(g) H2(g)+ I2(g)��ƽ���, �� �� > < �� =��
�ش���������:
H2(g)+ I2(g)��ƽ���, �� �� > < �� =��
�ش���������:
�ӷ�Ӧ��ʼ���ﵽƽ������Ҫʱ��: tA _______ tB����>��<�� =, ��ͬ��
ƽ��ʱ�ĵ��Ũ��: c(I2 )A c(I2 )B
HI��ƽ��ֽ�ٷ���Ϊ:  .
.
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com