
�������������������ʴ��ÿ����ʴ����ʧ�ĸֲ�ռ�������
�������1/4��
��1��������ʴ��Ҫ��������ʴ���ø�ʴ�����еĵ缫��Ӧʽ��
������ �������� ��
��2��Ϊ�˽���ijˮ�����բ�ű���ʴ�����ʣ����Բ�����ͼ��ʾ�ķ��������к�������բ�ŵĹ������R���Բ��� ������д������������ƣ�
��3����ͼ���ҷ���Ҳ�ɽ�����բ�Ÿ�ʴ���ʣ�������բ��Ӧ��������ֱ����Դ
�� ����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 AgOH+H+
AgOH+H+ AgOH+H+
AgOH+H+
| ||
| ||

 ���ϵĹ������R���Բ���
���ϵĹ������R���Բ���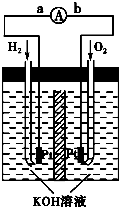 ����Ϊ
����Ϊ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������������������ʴ��ÿ����ʴ����ʧ�ĸֲ�ռ�������
�������1/4��
��1��������ʴ��Ҫ��������ʴ���ø�ʴ�����еĵ缫��Ӧʽ��
������ �������� ��
��2��Ϊ�˽���ijˮ�����բ�ű���ʴ�����ʣ����Բ�����ͼ��ʾ�ķ��������к�������բ�ŵĹ������R���Բ��� ������д������������ƣ�
��3����ͼ���ҷ���Ҳ�ɽ�����բ�Ÿ�ʴ���ʣ�������բ��Ӧ��������ֱ����Դ
�� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭�ճ����и߶���ѧ�ڵ��Ĵ��¿���ѧ�Ծ��������棩 ���ͣ������
�������������������ʴ��ÿ����ʴ����ʧ�ĸֲ�ռ���������������ķ�֮һ��

��1��Ϊ�˽���ijˮ�����բ�ű���ʴ�����ʣ����Բ���ͼ����ʾ�ķ��������к�������բ���ϵĹ������R���Բ���________(����ĸ)��
A��ͭ B����
C��п D��ʯī
��2��ͼ����ʾ�ķ���Ҳ���Խ�����բ�ŵĸ�ʴ���ʣ�������բ��Ӧ��������ֱ����Դ��________����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ̩���и߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
(8��)�������������������ʴ��
��1��������ʴ��Ҫ��������ʴ��������ʴ�����еĵ缫��ӦΪ��
����_______________ _______________��
����_______________________________ ��
��2��Ϊ�˽���ijˮ�����բ�ŵĸ�ʴ���ʣ����Բ���ͼ����ʾ�ķ��������к�������բ���ϵĹ������R���Բ��� _________(�����)��

A.ͭ B.�� C.п D.ʯī
��3��ͼ����ʾ�ķ���Ҳ���Խ�����բ�ŵĸ�ʴ���ʣ�������բ��Ӧ��������ֱ����Դ��___________���ϡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ�߶���ѧ��⻯ѧ�Ծ� ���ͣ������
��17�֣���1��AgNO3��ˮ��Һ�� ����ᡱ�����С���������ԣ�ԭ���ǣ������ӷ���ʽ��ʾ���� ��ʵ����������AgNO3����Һʱ������AgNO3���������ڽ�Ũ�� �У�Ȼ����������ˮϡ�͵������Ũ�ȡ����ö��Ե缫���AgNO3��ˮ��Һ����д����ط�Ӧ�����ӷ���ʽ
��2�������£�ij���Na2CO3����Һ�е����̪����Һ�ʺ�ɫ���ڷ�������Һ����̪�ʺ�ɫԭ��ʱ����ͬѧ��Ϊ��������Һ���õĴ�����Ʒ�л���NaOH��������ͬѧ��Ϊ����Һ��Na2CO3�������CO32��ˮ���������������һ����ʵ�鷽����������λͬѧ��˵�������У���������������ͽ��ۣ�
��3�������£�ȡpH=2������ʹ�����Һ��100mL�������зֱ����������Zn������Ӧ����������Һ��pH�仯��ͼ��ʾ����ͼ�б�ʾ������Һ��pH�仯���ߵ��� ���A����B�������������м����Zn����Ϊm1�� ������Һ�м����Zn����Ϊm2����m1 m2��ѡ���������=������������

��4���������������������ʴ��ÿ����ʴ����ʧ�ĸֲ�ռ���������������ķ�֮һ��
�ٸ�����ʴ��Ҫ��������ʴ���ø�ʴ�����еĵ缫��ӦʽΪ��
������
������ ��

��Ϊ�˽���ijˮ�����բ�ű���ʴ�����ʣ����Բ���ͼ����ʾ�ķ��������к�������բ���ϵĹ������R���Բ���________��
A��ͭ��������������B���� C��п D��ʯī
��ͼ����ʾ�ķ���Ҳ���Խ�����բ�ŵĸ�ʴ���ʣ�������բ��Ӧ��������ֱ����Դ��________����
��5������ȼ�ϵ���Ƿ�����ɫ��ѧ��������ͷ���װ�á���ͼΪ���ʾ��ͼ���õ�ص缫�����һ��ϸС�IJ��ۣ����������������ǿ�������ȶ�����ش�
 http://photo.blog.sina.com.cn/showpic.html
- blogid=515359c40100g2ml&url=http://static3.photo.sina.com.cn/orignal/515359c4g79a1c1001942&690
http://photo.blog.sina.com.cn/showpic.html
- blogid=515359c40100g2ml&url=http://static3.photo.sina.com.cn/orignal/515359c4g79a1c1001942&690
������ȼ�ϵ�ص�����ת����Ҫ��ʽ�� ���ڵ����е�����������Ϊ ����a��b��ʾ����
�ڸ�����ӦʽΪ ��
�۵缫����Ʋ��۵�ԭ��Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com