
��������ҽҩ��ʯ����ҵ���й㷺��;����ͼ��ģ�ҵ�Ʊ��������Ʒ�����Ƶ����̣�
��֪��Br2���ӷ��������ɫ��Һ�壻���������ӷ�����ɫҺ�塣
�����������̻ش��������⣺
��1����Ӧ�Ң��з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2����Ӧ�Ң�ʹ�ñ�ˮ��Ŀ�� ��
��3������I������ ���������õ��IJ��������� ��
��4����Ӧ�Ң��м���Na2SO3��Ŀ���� ��
��5����ҵ�������Ƶõ���������е����Ļ�ɫ�����Ǽ�����ͬѧ�����ʵ�����̽����
�ټ�ͬѧ���蹤ҵ������ʵ���ɫ����Ϊ��Fe3����������֤���ü������õ��Լ�Ϊ ������������ɹ۲쵽������Ϊ ��
����ͬѧ���蹤ҵ������ʵ���ɫ����Ϊ ��������֤���ü�������ķ���Ϊ ��
��1��SO2+Br2+2H2O=H2SO4+2HBr ��2����ֹBr2��HBr�ӷ�
��3������©�������������ձ��� ��4����ԭ��Ʒ�е�Br2
��5����KSCN��Һ����������KSCN��Һ���ɫ�����������к���Br2���ò�����պȡ�Ƶõ������ᣬ����ʪ�����KI��ֽ�ϱ��������ý�ͷ�ι�ȡ�Ƶõ����������Թ��У��μ�CCl4������ֹ���²�ʳȺ�ɫ����֤����Br2���Ի�ɫ��
���������������1����Ӧ�Ң���SO2��Br2�ڱ�ˮ�з�����Ӧ�Ļ�ѧ����ʽΪSO2+Br2+2H2O=H2SO4+2HBr����2����Ӧ�Ң�ʹ�ñ�ˮ��Ŀ����Ϊ�˷�ֹBr2��HBr�ӷ�����Ⱦ������Ӱ���������3������I�Ƿ��뻥�ܵķе㲻ͬ��Һ�����ʵIJ��������������������õ��IJ���������©�������������ձ�����4����Ӧ�Ң��м���Na2SO3��Ŀ����Ϊ������Ϊ��Ӧ��Br2��������ʵĴ��ȡ���5����ҵ�������Ƶõ���������е����Ļ�ɫ���ټ�ͬѧ���蹤ҵ������ʵ���ɫ����Ϊ��Fe3��������ķ�����ȡ��������Һ�������еμӼ���KSCN��Һ������ҵ��������KSCN��Һ���ɫ����֤������Fe3��������Ͱɺ���Fe3��������ͬѧ���蹤ҵ������ʵ���ɫ����Ϊ����Br2������ķ�����������ǿ�����ԡ��ò�����պȡ�Ƶõ������ᣬ����ʪ�����KI��ֽ�ϱ�����Ҳ�������������л��ܼ��е��ܽ�ȴ�����ʡ��ý�ͷ�ι�ȡ�Ƶõ����������Թ��У��μ�CCl4������ֹ���²�ʳȺ�ɫ����֤����Br2���Ի�ɫ��
���㣺����SO2��Br2�����ʡ������ķ��뷽����������Fe3����Br2�ļ��鷽����֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�Ӻ�������ȡ���ʵ������У�������ȷ�IJ�����



| A���������ճɻ� | B�����˺�I����Һ | C���ų���ı���Һ | D������Ⲣ���ձ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��10�֣�����ͼ��ʾA��F����������
��1��д���������ƣ�D ��E ��
��2�������˵������A����;�����ֻ��������ϣ� ��
��3��ʵ��������һ�����ʵ���Ũ�ȵ����ᣬ����36.5%��Ũ����4.5mL��ȡ��4.5mL����ʱ����Ҫ����ͼ�����е�F�� �����ţ���
��4������ʵ��������õ�����C���� ��ѡ������ѡ�����ĸ��ţ���
a������ˮ��CCl4�Ļ����
b������ˮ�;ƾ��Ļ����
c������ˮ����ɰ�Ļ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ����̼���̿�Ϊ��Ҫԭ������MnO2�Ĺ����������£� �й��������↑ʼ�����ͳ�����ȫ��pH���±���
�й��������↑ʼ�����ͳ�����ȫ��pH���±���
| �������� | Al��OH��2 | Fe��OH��3 | Fe��OH��2 | Cu��OH��2 | Pb��OH��2 | Mn��OH��2 |
| ��ʼ������pH | 3.3 | 1.5 | 6.5 | 4.2 | 8.0 | 8.3 |
| ������ȫ��pH | 5.2 | 3.7 | 9.7 | 6.7 | 8.8 | 9.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ѧ��ȤС������ij����������ͭп����ȡ����ZnOʵ���������£�
��ش��������⣺
��1���������ۺ�����Ӧ�����ӷ���ʽΪ__________��
��2���ס�����ͬѧѡ���������������ò�ͬ�ķ�������ȡ������
�ټ�ͬѧʹ�õ�ҩƷ����ʯ�����Ȼ�泥���Ӧѡ��װ��______����дװ�ô��ţ������ɰ����Ļ�ѧ����ʽΪ____________��
����ͬѧѡ����װ��B����ʹ�õ�����ҩƷ������Ϊ_______________��
��3��H2O2��������_____________��
��4�����������еõ���Fe(OH)3����KClO��Һ�ڼ��Ի������������õ�һ�ָ�Ч�Ķ��ˮ��������K2FeO4�����÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ____________��
��5����֪��Һa�к���CO32-��SO42-������������ӣ���ֻ����ȡ��һ����Ʒ�������������Ӵ��ڵ�ʵ���������Ϊ___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������±ʯ(K2SO4��MgSO4��2CaSO4��2H2O)���ڡ�������ˮ�д�������ƽ�⣺
K2SO4��MgSO4��2CaSO4��2H2O(s)  2Ca2����2K����Mg2����4
2Ca2����2K����Mg2����4 ��2H2O
��2H2O
Ϊ�ܳ�����ü���Դ���ñ���Ca(OH)2��Һ�ܽ���±ʯ�Ʊ�����أ������������£�
(1)������Ҫ�ɷ���________��________�Լ�δ����±ʯ��
(2)�û�ѧƽ���ƶ�ԭ������Ca(OH)2��Һ���ܽ���±ʯ����K����ԭ��_________________________________________________��
(3)�����ӡ������У��ȼ���________��Һ��������Ȳ������ˣ��ټ���________��Һ����ҺpH�����ԡ�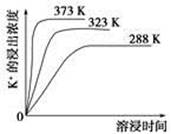
(4)��ͬ�¶��£�K���Ľ���Ũ�����ܽ�ʱ��Ĺ�ϵ��ͼ����ͼ�ɵã������¶����ߣ�
��________________________________________________________��
��________________________________________________________��
(5)�����Կ�����̼����Ϊ�ܽ��������ܽ������лᷢ����CaSO4(s)�� ?
? CaCO3(s)��
CaCO3(s)��
��֪298 Kʱ��Ksp(CaCO3)��2.80��10��9��
Ksp(CaSO4)��4.90��10��5������¶��¸÷�Ӧ��ƽ�ⳣ��K(������������λ��Ч����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ң���Ҫ�ɷ�ΪAl��Al2O3����������CuO��SiO2��FeO��Fe2O3���ʣ�Ϊԭ�ϣ����Ƶ�Һ��ۺ��Ȼ���Alm��OH��nCl3m-n�������IJ��ֹ�������ͼ��ʾ�����ֲ���Ͳ�������ȥ����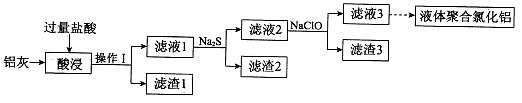
��֪ijЩ������������±���
��1������I�� ��Al2O3�����ᷴӦ�����ӷ���ʽ�� ��
��2������2Ϊ��ɫ���ú�ɫ���ʵĻ�ѧʽ�� ��
��3������Һ2�м���NaClO��Һ�����ٲ������ɫ��������ʱ��Һ��pHԼΪ3��7��NaClO�������� ��
��4������Һ3��pH����4��2��4��5������ˮ�ⷴӦ�õ�Һ��ۺ��Ȼ�������Ӧ�Ļ�ѧ����ʽ�� ��
��5������Һ3���Ҳ���Եõ�Һ��ۺ��Ȼ�����װ����ͼ��ʾ�������ӽ���Ĥֻ����������ͨ�����缫Ϊ���Ե缫����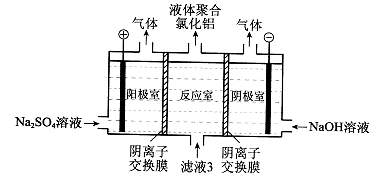
��д�������ҵĵ缫��Ӧ�� ��
�ڼ����ڷ�Ӧ�������ɾۺ���������ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ���������̿�������������Һ��ȡ����̣�����������Һ�Ʊ��ߴ�̼���̡���������淋Ĺ�����������(���̿����Ҫ�ɷ���MnO2�������й衢��������������������ؽ��������������)��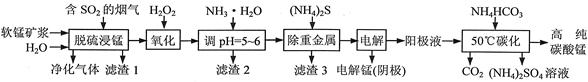
��1��һ���¶��£���������̡���Ҫ����ΪMnSO4���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��2��������2������Ҫ�ɷֵĻ�ѧʽΪ ��
��3�������ؽ�����ʱʹ��(NH4)2S����ʹ��Na2S��ԭ���� ��
��4������⡱ʱ�ö��Ե缫�������ĵ缫��ӦʽΪ ��
��5����50��̼�����õ��ߴ�̼���̣���Ӧ�����ӷ���ʽΪ ����50��̼����ʱ�������NH4HCO3�����ܵ�ԭ���ǣ�ʹMnSO4���ת��ΪMnCO3�� �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ˮ������ҵ��ɰ�ǡ���֬��Ư����ɱ�������У���������(NaClO2)��������Ҫ�����á���ͼ�������������ƵĹ�������ͼ��
��֪����NaClO2���ܽ�����¶����߶������ʵ������¿ɽᾧ����NaClO2��3H2O��
�ڳ����£�Ksp(FeS)=6��3��10-18��Ksp(CuS)=6��3��10-28��Ksp(PbS)=2��4 ��10-28
��1����ӦI�з�����Ӧ�����ӷ���ʽΪ ��
��2������Һ�еõ�NaClO2��3H2O������������������ (��д���)��
a������ b������Ũ�� c������ d����ȴ�ᾧ e������
��3��ӡȾ��ҵ������������(NaClO2)Ư��֯�Ư��֯��ʱ���������õ���HClO2���±���25��ʱHClO2�����ֳ�������ĵ���ƽ�ⳣ����
| ���� | HClO2 | HF | H2CO3 | H2S |
| Ka��mol��L-1 | 1��10-2 | 6.3��10-4 | K1=4.30��10-7 K2=5.60��10-11 | K1=9.1��10-8 K2=l.1��10-12 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com