
���� ��һ����Һ��֪���п��ܺ���Mg2+��Cu2+��Fe2+��Al3+��NH4+��K+��Cl-��HCO3-��SO42-��������һ�ֵ���ɫ��ĩ״��������ʱ���д̼�����ζ�Ļ������ų���ͬʱ���ɰ�ɫ������˵����Һ��һ��������Cu2+��Fe2+���ƶϵ���ɫ�����ĩΪ�������ƣ���ˮ��Ӧ�����������ƺ��������̼�����ζ�Ļ������ΪNH3��O2��˵������NH4+��������0.4mol����ɫ��ĩʱ�����ռ���0.3mol������壬2Na2O2+2H2O=4NaOH+O2����OH-+NH4+=NH3•H2O������������Ϊ0.2mol�����ɰ���Ϊ0.1mol�����������������ƺ�笠���Ӧ��Ϊ0.1mol�����ɳ�����Ӧ�������������ʵ���Ϊ0.7mol���Ҵ�ʱ���ɵij�����࣮�˺�������뵭��ɫ��ĩʱ�����������٣�������0.45mol��ĩ��������0.3mol������0.2mol��֤��һ������Al3+��˵����Һ��һ��������HCO3-����Al3+���ʵ���Ϊ0.1mol��Mg2+Ϊ0.2mol�����ɳ���ΪMg��OH��2����ԭ��Һ��һ������Mg2+��Al3+��NH4+��һ��������Cu2+��Fe2+��HCO3-�����ܺ���K+��Cl-��SO42-�е�һ�ֻ��֣��Դ˽����⣮
��� �⣺��һ����Һ��֪���п��ܺ���Mg2+��Cu2+��Fe2+��Al3+��NH4+��K+��Cl-��HCO3-��SO42-��������һ�ֵ���ɫ��ĩ״��������ʱ���д̼�����ζ�Ļ������ų���ͬʱ���ɰ�ɫ������˵����Һ��һ��������Cu2+��Fe2+���ƶϵ���ɫ�����ĩΪ�������ƣ���ˮ��Ӧ�����������ƺ��������̼�����ζ�Ļ������ΪNH3��O2��˵������NH4+��������0.4mol����ɫ��ĩʱ�����ռ���0.3mol������壬2Na2O2+2H2O=4NaOH+O2����OH-+NH4+=NH3•H2O������������Ϊ0.2mol�����ɰ���Ϊ0.1mol�����������������ƺ�笠���Ӧ��Ϊ0.1mol�����ɳ�����Ӧ�������������ʵ���Ϊ0.7mol���Ҵ�ʱ���ɵij�����࣮�˺�������뵭��ɫ��ĩʱ�����������٣�������0.45mol��ĩ��������0.3mol������0.2mol��֤��һ������Al3+��˵����Һ��һ��������HCO3-����Al3+���ʵ���Ϊ0.1mol��Mg2+Ϊ0.2mol�����ɳ���ΪMg��OH��2����ԭ��Һ��һ������Mg2+��Al3+��NH4+��һ��������Cu2+��Fe2+��HCO3-�����ܺ���K+��Cl-��SO42-�е�һ�ֻ��֣�
��1������ɫ��ĩ������Ϊ�������ƣ�
�ʴ�Ϊ���������ƣ�
��2�����ݷ����ж�ԭ��Һ��һ������Mg2+��Al3+��NH4+��һ��������Cu2+��Fe2+��HCO3-����Һ�����Կ�֪�����ܺ���K+��Cl-��SO42-�е�һ�ֻ��֣�
�ʴ�Ϊ��NH4+��Al3+��Mg2+��Cu2+��Fe2+��HCO3-�� K+��Cl-��SO42-��
��3���ٵ��Ʒ�ĩ��ˮ��Ӧ�����������ƺ���������Ӧ�Ļ�ѧ����ʽΪ��2Na2O2+2H2O=4NaOH+O2�����ʴ�Ϊ��2Na2O2+2H2O=4NaOH+O2����
�ڲ����̼�����ζ��������ӷ���ʽOH-+NH4+=NH3��+H2O���ʴ�Ϊ��OH-+NH4+=NH3��+H2O��
�ۼ��뵭��ɫ��ĩ�����ʵ�����0.4mol��0.45molʱ������������ʧ�����������ܽ�������������Һ�У���Ӧ�����ӷ���ʽΪ��Al��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ����2Na2O2+2H2O=4NaOH+O2���� ��NH4++OH-=NH3��+H2O�� ��Al��OH��3+OH-=AlO2-+2H2O��
��4�������Ϸ�����֪��Һ�������ӵ�������֮��Ϊn��NH4+����n��Mg2+����n��Al3+��=0.1mol��0.2mol��0.1mol=1��2��1��
�ʴ�Ϊ��NH4+��Mg2+��Al3+=0.1��0.2��0.1=1��2��1��
���� ���⿼���������ƶϡ���Ӧ�Ķ�������ȣ�Ϊ��Ƶ���㣬������ѧ���ķ��������������Ŀ��飬��Ŀ�ۺ��Ժ�ǿ���������ӷ�Ӧ���������ʵ������Ͷ�����ϵ�����жϺ��е������ǹؼ����ѶȺܴ�ע�ʼ�μ��ȣ��ᵼ�°����ݳ���


 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
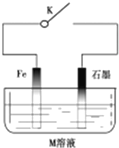
| A�� | �����£��Ͽ�Kʱ����M��ҺΪŨ���ᣬ����������ѧ��ʴ | |
| B�� | �ر�K����MΪ�Ȼ��ƣ��������ĵ缫��ӦʽΪO2+4e-+2H2O=4OH- | |
| C�� | �ر�K����MΪ����泥���ʯī���ĵ缫��ӦʽΪ2H++2e-=H2�� | |
| D�� | �ر�K����M��ҺΪ��ˮ��ʯī����ͭ�����������ӵ��������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 4Na+O2�T2Na2O | B�� | NH4HCO3�TNH3��+H2O+CO2�� | ||
| C�� | NH3+HCl�TNH4C1 | D�� | H2SO4+2NaOH�TNa2SO4+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢ� | B�� | �ڢۢ� | C�� | �٢ۢ� | D�� | �ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 12C��14CΪ��ͬ���� | B�� | ʯī��C60��Ϊͬ�������� | ||
| C�� | H2O��D2O��Ϊͬλ�� | D�� | ���������黥Ϊͬϵ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

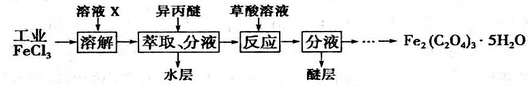
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com