
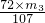 ������CuO��������m1-m2-
������CuO��������m1-m2-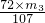 ��
�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



m1-m2-
| ||
| m1 |
m1-m2-
| ||
| m1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
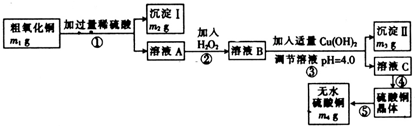
Ϊ�ⶨ������ͭ�����к���������������������������ʣ���CuO����������������ȡ��ˮ����ͭ��ij��ѧ�С�����������ʵ�飺
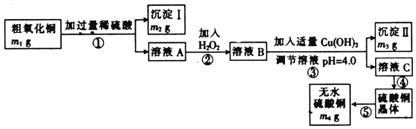
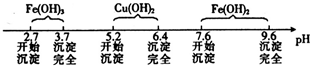
��֪Fe3+��Cu2+��Fe2+������������Һ���γ��������������pH��Χ���£�
��ش��������⣺
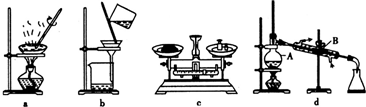
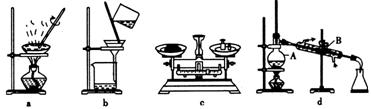
��1��������ʵ������У�����ʵ��װ�ò������õ����� ������ĸ����װ��d������A��B�����Ʒֱ�Ϊ ��
��2����ҺA���������ʵĻ�ѧʽΪ ��
��3������ڷ�����Ӧ�����ӷ���ʽΪ ��
��4������II�Ļ�ѧʽΪ ��
��5��������ͭ��Ʒ��CuO����������Ϊ �����г�����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ�ⶨ������ͭ�����к���������������������������ʣ���CuO����������������ȡ��ˮ����ͭ��ij��ѧ�С�����������ʵ�飺
��֪Fe3+��Cu2+��Fe2+������������Һ���γ��������������pH��Χ���£�
��ش��������⣺
��1��������ʵ������У�����ʵ��װ�ò������õ����� ������ĸ����װ��d������A��B�����Ʒֱ�Ϊ ��
��2����ҺA���������ʵĻ�ѧʽΪ ��
��3������ڷ�����Ӧ�����ӷ���ʽΪ ��
��4������II�Ļ�ѧʽΪ ��
��5��������ͭ��Ʒ��CuO����������Ϊ �����г�����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ӱ�ʡ��ɽһ�и�����ѧ�ڵ�һ�ε��п��ԣ����ۣ���ѧ���� ���ͣ�ʵ����
Ϊ�ⶨ������ͭ�����к���������������������������ʣ���CuO�������������� ��ȡ��ˮ����ͭ��ij��ѧ�С�����������ʵ�飺
��ȡ��ˮ����ͭ��ij��ѧ�С�����������ʵ�飺
��֪Fe3+��Cu2+��Fe2+������������Һ���γ��������������pH��Χ���£�
��ش��������⣺
��1��������ʵ������У�����ʵ��װ�ò������õ����� ������ĸ����װ��d������A��B�����Ʒֱ�Ϊ ��
��2����ҺA���������ʵĻ�ѧʽΪ ��
��3������ڷ�����Ӧ�����ӷ���ʽΪ ��
��4������II�Ļ�ѧʽΪ ��
��5��������ͭ��Ʒ��CuO����������Ϊ �����г�����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��ӱ�ʡ������ѧ�ڵ�һ�ε��п��ԣ����ۣ���ѧ���� ���ͣ�ʵ����
Ϊ�ⶨ������ͭ�����к���������������������������ʣ���CuO����������������ȡ��ˮ����ͭ��ij��ѧ�С�����������ʵ�飺

��֪Fe3+��Cu2+��Fe2+������������Һ���γ��������������pH��Χ���£�

��ش��������⣺
��1��������ʵ������У�����ʵ��װ�ò������õ����� ������ĸ����װ��d������A��B�����Ʒֱ�Ϊ ��

��2����ҺA���������ʵĻ�ѧʽΪ ��
��3������ڷ�����Ӧ�����ӷ���ʽΪ ��
��4������II�Ļ�ѧʽΪ ��
��5��������ͭ��Ʒ��CuO����������Ϊ �����г�����ʽ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com