
”¾ĢāÄæ”æĀČ»ÆŃĒĶŹĒÖŲŅŖµÄĶŃĪĻµĮŠ»Æ¹¤²śĘ·£¬¹ć·ŗÓ¦ÓĆÓŚŹÆÓĶ»Æ¹¤”¢ÓŠ»śŗĻ³ÉµČŠŠŅµ”£CuCl¾§Ģå³Ź°×É«£¬¼ū¹āŅ×·Ö½ā£¬Ī¢ČÜÓŚĖ®£¬²»ČÜÓŚĻ”ŃĪĖįŗĶŅŅ“¼£¬Ā¶ÖĆÓŚ³±ŹŖæÕĘųÖŠŅ×Ė®½āŃõ»ÆĪŖĀĢÉ«µÄ![]() ”£Ä³ŃŠ¾æŠ”×éŅŌ
”£Ä³ŃŠ¾æŠ”×éŅŌ![]() (ŗ¬ÉŁĮæ
(ŗ¬ÉŁĮæ![]() )“ÖĘ·ĪŖŌĮĻÖĘČ”CuCl£¬Éč¼ĘµÄŗĻ³ÉĀ·ĻßČēĻĀ£ŗ
)“ÖĘ·ĪŖŌĮĻÖĘČ”CuCl£¬Éč¼ĘµÄŗĻ³ÉĀ·ĻßČēĻĀ£ŗ
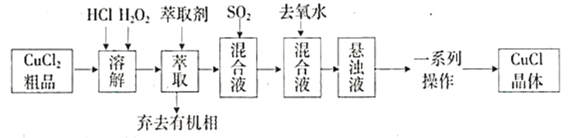
ŅŃÖŖ£ŗ¢ŁŌŚ½ĻøßÅØ¶ČµÄŃĪĖįĻĀ£¬![]() ÄÜČܽāÓŚ¼×»łŅģ¶”»ł¼×ĶŖ”£
ÄÜČܽāÓŚ¼×»łŅģ¶”»ł¼×ĶŖ”£
¢ŚCuClŌŚČÜŅŗÖŠ“ęŌŚ£ŗ![]()
(1)ÉĻŹöŗĻ³ÉĀ·ĻßÖŠ£¬![]() µÄ×÷ÓĆŹĒ________________________£¬ŻĶČ”¼ĮĪŖ¼×»łŅģ¶”»ł¼×ĶŖ£¬Ęä×÷ÓĆŹĒ________________”£
µÄ×÷ÓĆŹĒ________________________£¬ŻĶČ”¼ĮĪŖ¼×»łŅģ¶”»ł¼×ĶŖ£¬Ęä×÷ÓĆŹĒ________________”£
(2)ÉĻŹöŗĻ³ÉĀ·ĻßÖŠ£¬![]() ĶØČė»ģŗĻŅŗµÄŹµŃé×°ÖĆČēĶ¼ĖłŹ¾£ŗ
ĶØČė»ģŗĻŅŗµÄŹµŃé×°ÖĆČēĶ¼ĖłŹ¾£ŗ
¢Ł×°ÖĆB”¢DµÄ×÷ÓĆ·Ö±šŹĒ________________________________________________”£
¢ŚCÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ___________________________________”£
(3)ÉĻŹöŗĻ³ÉĀ·ĻßÖŠ£¬Ļņ»ģŗĻŅŗÖŠ¼ÓČė“óĮæČ„ŃõĖ®µÄÄæµÄŹĒ______________________”£
(4)ÉĻŹöŗĻ³ÉĀ·ĻßÖŠ£¬Ņ»ĻµĮŠ²Ł×÷°üĄØ£ŗ³éĀĖ”¢Ļ“µÓ”¢øÉŌļ”£
øÉŌļŹ±Ó¦×¢ŅāĆÜ·ā”¢_________________________”£
”¾“š°ø”潫ČÜŅŗÖŠ![]() Ńõ»ÆĪŖ
Ńõ»ÆĪŖ![]() ³żČ„
³żČ„![]() ·ĄÖ¹µ¹Īü”¢ĪüŹÕ
·ĄÖ¹µ¹Īü”¢ĪüŹÕ![]() Ī²Ęų£¬·ĄÖ¹ĪŪČ¾»·¾³
Ī²Ęų£¬·ĄÖ¹ĪŪČ¾»·¾³ ![]() Ź¹Ę½ŗā
Ź¹Ę½ŗā![]() ÄęĻņŅĘ¶Æ£¬Éś³ÉCuC1³Įµķ ±Ü¹ā
ÄęĻņŅĘ¶Æ£¬Éś³ÉCuC1³Įµķ ±Ü¹ā
”¾½āĪö”æ
![]() (ŗ¬ÉŁĮæ
(ŗ¬ÉŁĮæ![]() )“ÖĘ·¼ÓČėH2O2£¬H2O2°Ń
)“ÖĘ·¼ÓČėH2O2£¬H2O2°Ń![]() Ńõ»ÆĪŖ
Ńõ»ÆĪŖ![]() £¬¼ÓČėŻĶČ”¼Į¼×»łŅģ¶”»ł¼×ĶŖ³żČ„
£¬¼ÓČėŻĶČ”¼Į¼×»łŅģ¶”»ł¼×ĶŖ³żČ„![]() £¬ÓĆSO2°Ń
£¬ÓĆSO2°Ń![]() »¹ŌĪŖ
»¹ŌĪŖ![]() £¬¼ÓČėČ„ŃõĖ®£¬
£¬¼ÓČėČ„ŃõĖ®£¬![]() ÄęĻņŅĘ¶Æ£¬Éś³ÉCuC1³Įµķ”£
ÄęĻņŅĘ¶Æ£¬Éś³ÉCuC1³Įµķ”£
(1)![]() (ŗ¬ÉŁĮæ
(ŗ¬ÉŁĮæ![]() )“ÖĘ·¼ÓČėH2O2£¬H2O2°Ń
)“ÖĘ·¼ÓČėH2O2£¬H2O2°Ń![]() Ńõ»ÆĪŖ
Ńõ»ÆĪŖ![]() £¬±ćÓŚŻĶČ”³żČ„ĢśŌŖĖŲ£¬
£¬±ćÓŚŻĶČ”³żČ„ĢśŌŖĖŲ£¬![]() µÄ×÷ÓĆŹĒ½«ČÜŅŗÖŠ
µÄ×÷ÓĆŹĒ½«ČÜŅŗÖŠ![]() Ńõ»ÆĪŖ
Ńõ»ÆĪŖ![]() £¬ŌŚ½ĻøßÅØ¶ČµÄŃĪĖįĻĀ£¬
£¬ŌŚ½ĻøßÅØ¶ČµÄŃĪĖįĻĀ£¬![]() ÄÜČܽāÓŚ¼×»łŅģ¶”»ł¼×ĶŖ£¬¼ÓČėŻĶČ”¼ĮĪŖ¼×»łŅģ¶”»ł¼×ĶŖ£¬Ęä×÷ÓĆŹĒ³żČ„
ÄÜČܽāÓŚ¼×»łŅģ¶”»ł¼×ĶŖ£¬¼ÓČėŻĶČ”¼ĮĪŖ¼×»łŅģ¶”»ł¼×ĶŖ£¬Ęä×÷ÓĆŹĒ³żČ„![]() £»
£»
(2)¢Ł×°ÖĆBĮ½²ąµ¼¹ÜøÕĀ¶³öĻš½ŗČū£¬BµÄ×÷ÓĆŹĒ·ĄÖ¹µ¹Īü£¬¶žŃõ»ÆĮņÓŠ¶¾£¬ÄÜĪŪČ¾æÕĘų£¬DÖŠŹ¢·ÅĒāŃõ»ÆÄĘČÜŅŗĪüŹÕ¶žŃõ»ÆĮņ£¬·ĄÖ¹ĪŪČ¾»·¾³£»
¢ŚCÖŠ¶žŃõ»ÆĮņ°Ń![]() »¹ŌĪŖ
»¹ŌĪŖ![]() £¬øł¾ŻµĆŹ§µē×ÓŹŲŗć”¢Ō×ÓŹŲŗć”¢µēŗÉŹŲŗć£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ
£¬øł¾ŻµĆŹ§µē×ÓŹŲŗć”¢Ō×ÓŹŲŗć”¢µēŗÉŹŲŗć£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ![]() £»
£»
(3) CuClŌŚČÜŅŗÖŠ“ęŌŚ£ŗ![]() £¬Ļņ»ģŗĻŅŗÖŠ¼ÓČė“óĮæČ„ŃõĖ®£¬Ź¹Ę½ŗā
£¬Ļņ»ģŗĻŅŗÖŠ¼ÓČė“óĮæČ„ŃõĖ®£¬Ź¹Ę½ŗā![]() ÄęĻņŅĘ¶Æ£¬Éś³ÉCuC1³Įµķ£»
ÄęĻņŅĘ¶Æ£¬Éś³ÉCuC1³Įµķ£»
(4) CuCl¾§Ģå³Ź°×É«£¬¼ū¹āŅ×·Ö½ā£¬Ā¶ÖĆÓŚ³±ŹŖæÕĘųÖŠŅ×Ė®½āŃõ»ÆĪŖĀĢÉ«µÄ![]() £¬øÉŌļŹ±Ó¦×¢ŅāĆÜ·ā”¢±Ü¹ā”£
£¬øÉŌļŹ±Ó¦×¢ŅāĆÜ·ā”¢±Ü¹ā”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
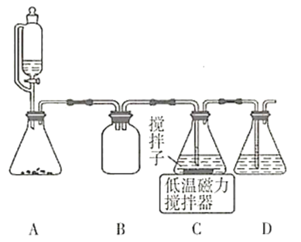
”¾ĢāÄæ”æÓĆĻĀĶ¼ĖłŹ¾×°ÖĆ½ųŠŠČēĻĀŹµŃ飬ÄÜ“ļµ½ŹµŃéÄæµÄµÄŹĒ

A. ĘæÖŠŹ¢ĀśĖ®£¬“ÓBæŚ½ųĘų£¬ÓĆÅÅĖ®·ØŹÕ¼ÆHClĘųĢå
B. ĘæÖŠŹ¢ŹŹĮæÅØĮņĖį£¬“ÓAæŚ½ųĘųĄ“øÉŌļNH3
C. “ÓBæŚ½ųĘų£¬ÓĆÅÅæÕĘų·ØŹÕ¼ÆCO2
D. ĘæÖŠŹ¢ĀśĖ®£¬AæŚĮ¬µ¼¹Ü²¢ÉģČėĮæĶ²ÖŠ£¬“ÓBæŚ½ųĘų£¬ÓĆÅÅĖ®·Ø²āĮæÉś³ÉH2µÄĢå»ż
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ׶ŠĪĘæ֊װӊ²æ·Ö±äÖŹµÄĘÆ·Ū¾«·ŪÄ©ŗĶŗģÉ«Ö½»Ø£¬ĻņĘä֊עÉäÅØŃĪĖį£¬¹Ū²ģµ½Ö½»ØĶŹÉ«£¬²¢ÓŠ»ĘĀĢÉ«ĘųĢåÉś³É£¬øĆŹµŃéæÉŅŌµĆµ½µÄ½įĀŪŹĒ( )
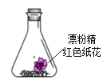
A.ĖµĆ÷![]() ¾ßÓŠĘư׊ŌB.ŹµŃéÖŠÖ»·¢ÉśĮĖŃõ»Æ»¹Ō·“Ó¦
¾ßÓŠĘư׊ŌB.ŹµŃéÖŠÖ»·¢ÉśĮĖŃõ»Æ»¹Ō·“Ó¦
C.ÅØŃĪĖį±»Ńõ»ÆD.·“Ó¦ÖŠÉś³ÉµÄĘųĢåÖ»ÓŠ![]()
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠ·“Ó¦ÖŠ£¬²»ŹĒŃõ»Æ»¹Ō·“Ó¦µÄŹĒ ( )
¢ŁH2+Cl2![]() 2HCl
2HCl
¢ŚNa2CO3+2HCl=2NaCl+H2O+CO2”ü
¢Ū2H2O![]() 2H2”ü+O2”ü
2H2ӟ+O2ӟ
¢ÜCuO+2HNO3=Cu(NO3)2+H2O
¢Ż2HgO![]() 2Hg+O2”ü
2Hg+O2ӟ
A.¢Ś¢ÜB.¢Ł¢ŪC.¢Ł¢Ś¢ŪD.¢Ü¢Ż
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŹµŃéŹŅÖʱø²¢ŹÕ¼ÆøÉŌļ”¢“æ¾»ĀČĘųµÄ×°ÖĆČēĶ¼ĖłŹ¾£ŗ
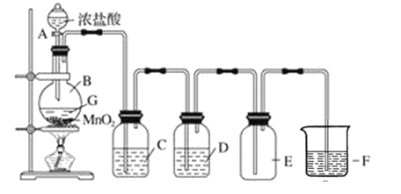
£Ø1£©Š“³öŹµŃéŹŅÖʱøCl2µÄ»Æѧ·½³ĢŹ½£ŗ________________________”£
£Ø2£©Š“³öÖø¶ØŹŌ¼ĮµÄĆū³Ę£¬C_____________________£¬D_______________________”£
£Ø3£©CµÄ×÷ÓĆŹĒ_______________£¬DµÄ×÷ÓĆŹĒ_______________£¬FµÄ×÷ÓĆŹĒ____________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠ¹ŲÓŚÓŠ»ś»ÆŗĻĪļµÄĖµ·Ø“ķĪóµÄŹĒ( )
A.ŅŅĻ©”¢¾ŪĀČŅŅĻ©ŗĶ±½ÖŠ¾łŗ¬ÓŠĢ¼Ģ¼Ė«¼ü
B.Ö²ĪļÓĶŗ¬²»±„ŗĶøß¼¶Ö¬·¾Ėįõ„£¬ÄÜŹ¹![]() µÄ
µÄ![]() ČÜŅŗĶŹÉ«
ČÜŅŗĶŹÉ«
C.ŅŅĖįŅŅõ„ÖŠ»ģÓŠµÄÉŁĮæŅŅĖį£¬æÉÓƱ„ŗĶ![]() ČÜŅŗ³żČ„
ČÜŅŗ³żČ„
D.ÓĆ¾Ę¾«Ļū¶¾£¬ĘäŌĄķŹĒ¾Ę¾«Ź¹Ļø¾śÖŠµÄµ°°×ÖŹ±äŠŌ¶ųŹ§Č„ÉśĄķ»īŠŌ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æōĒ°±![]() ŌŚĖ®ČÜŅŗÖŠµÄµēĄė·½³ĢŹ½ĪŖ£ŗ
ŌŚĖ®ČÜŅŗÖŠµÄµēĄė·½³ĢŹ½ĪŖ£ŗ![]() £¬ÓĆ0.1mol/LŃĪĖįµĪ¶Ø20mL 0.1mol/L
£¬ÓĆ0.1mol/LŃĪĖįµĪ¶Ø20mL 0.1mol/L![]() ČÜŅŗ£¬ŗć¶Ø25”ꏱ£¬µĪ¶Ø¹ż³ĢÖŠÓÉĖ®µēĄė³öĄ“µÄOH”„ÅØ¶ČµÄøŗ¶ŌŹżÓėŃĪĖįĢå»żµÄ¹ŲĻµČēĶ¼ĖłŹ¾£¬ĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ( )
ČÜŅŗ£¬ŗć¶Ø25”ꏱ£¬µĪ¶Ø¹ż³ĢÖŠÓÉĖ®µēĄė³öĄ“µÄOH”„ÅØ¶ČµÄøŗ¶ŌŹżÓėŃĪĖįĢå»żµÄ¹ŲĻµČēĶ¼ĖłŹ¾£¬ĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ( )

A.Bµć¶ŌÓ¦µÄČÜŅŗÖŠ![]()
B.Cµć¶ŌÓ¦µÄČÜŅŗÖŠ![]()
C.Aµ½CČÜŅŗÖŠ£¬![]() µēĄė³£Źż²»±ä
µēĄė³£Źż²»±ä
D.ÓÉAµ½D£¬Ė®µēĄė³öµÄ![]() ĻČ¼õŠ”ŗóŌö“ó
ĻČ¼õŠ”ŗóŌö“ó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ0.096 kgĢ¼ĶźČ«Č¼ÉÕÉś³ÉCO2ĘųĢåæɷųö3147.9 kJµÄČČĮ棬ŌņĻĀĮŠČČ»Æѧ·½³ĢŹ½ÕżČ·µÄŹĒ( )
A. C(s)£«O2(g)===CO2(g) ¦¤H£½£393.49 kJ/mol
B. C(s)£«O2(g)===CO2(g) ¦¤H£½£«393.49 kJ/mol
C. C£«O2===CO2 ¦¤H£½£393.49 kJ/mol
D. C(s)£«![]() O2(g)===CO(g) ¦¤H£½£393.49 kJ/mol
O2(g)===CO(g) ¦¤H£½£393.49 kJ/mol
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æČżĀČ»ÆĮł°±ŗĻīÜ([Co(NH3)6]Cl3)ŹĒŅ»ÖÖ³Č»ĘÉ«¾§Ģ壬ŹµŃéŹŅÖʱø¹ż³ĢČēĻĀ£ŗ
¢ń£®½«ŃŠĻøµÄ6 g CoCl26H2O¾§ĢåŗĶ4 g NH4Cl¹ĢĢå¼ÓČė׶ŠĪĘæÖŠ£¬¼ÓĖ®£¬¼ÓČČČܽā£¬ĄäČ“£»
¢ņ£®¼ÓČė13.5 mLÅØ°±Ė®£¬ÓĆ»īŠŌĢæ×÷“߻ƼĮ£¬»ģŗĻ¾łŌČŗóÖšµĪµĪ¼Ó13.5 mL 5% H2O2ČÜŅŗ£¬Ė®Ō”¼ÓČČÖĮ50~60”ę£¬±£³Ö20 min”£ÓƱłŌ”ĄäČ“£¬¹żĀĖ£¬µĆ“Ö²śĘ·£»
¢ó£®½«“Ö²śĘ·ČÜÓŚ50 mLČȵÄĻ”ŃĪĖįÖŠ£¬______£¬ĻņĀĖŅŗÖŠ»ŗĀż¼ÓČė6.7 mLÅØŃĪĖį£¬ÓŠ“óĮæ³Č»ĘÉ«¾§ĢåĪö³ö£¬±łŌ”ĄäČ“ŗó¹żĀĖ£»
¢ō£®ĻČÓĆĄäµÄ2 mol”¤L1 HClČÜŅŗĻ“µÓ¾§Ģ壬ŌŁÓĆÉŁŠķŅŅ“¼Ļ“µÓ£¬øÉŌļ£¬µĆ²śĘ·”£
(1)[Co(NH3)6]Cl3ÖŠCoµÄ»ÆŗĻ¼ŪŹĒ______”£
(2)CoCl2ÓöÅØ°±Ė®Éś³ÉCo(OH)2³Įµķ£¬¼ÓČėÅØ°±Ė®Ē°ĻČ¼ÓČėNH4ClæɱÜĆā³ĮµķÉś³É£¬ŌŅņŹĒ______”£
(3)ČÜŅŗÖŠCoCl2”¢NH4ClŗĶÅØ°±Ė®»ģŗĻŗó£¬ÓėH2O2ČÜŅŗ·“Ӧɜ³É[Co(NH3)6]Cl3µÄ»Æѧ·½³ĢŹ½ŹĒ______”£
(4)²¹Č«¢óÖŠµÄ²Ł×÷£ŗ______”£
(5)³ĮµķµĪ¶Ø·Ø²ā¶ØÖʱøµÄ²śĘ·ÖŠCl£µÄÖŹĮæ·ÖŹż£ŗ
¢”£®×¼Č·³ĘČ”a g ¢ōÖŠµÄ²śĘ·£¬ÅäÖĘ³É100 mLČÜŅŗ£¬ŅĘČ”25 mLČÜŅŗӌ׶ŠĪĘæÖŠ£»
¢¢£®µĪ¼ÓÉŁĮæ0.005 mol”¤L1 K2CrO4ČÜŅŗ×÷ĪŖÖøŹ¾¼Į£¬ÓĆc mol”¤L1 AgNO3ČÜŅŗµĪ¶ØÖĮÖÕµć£»
¢££®Ę½ŠŠ²ā¶ØČż“Ī£¬ĻūŗÄAgNO3ČÜŅŗµÄĢå»żµÄĘ½¾łÖµĪŖv mL£¬¼ĘĖć¾§ĢåÖŠCl£µÄÖŹĮæ·ÖŹż”£
ŅŃÖŖ£ŗČܽā¶Č£ŗAgCl 1.3”Į106 mol”¤L1£¬Ag2CrO4(שŗģÉ«)6.5”Į105 mol”¤L1
¢Ł¢¢ÖŠ£¬µĪ¶ØÖĮÖÕµćµÄĻÖĻóŹĒ______”£
¢ŚÖʱøµÄ¾§ĢåÖŠCl£µÄÖŹĮæ·ÖŹżŹĒ______(ĮŠ¼ĘĖćŹ½£¬ClµÄĻą¶ŌŌ×ÓÖŹĮæ£ŗ35.5)”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com