
| 0.4g |
| 0.01mol |
| 0.4g |
| 0.01mol |
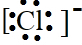 ��
��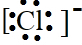 ��
��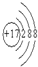 ��̼ԭ�Ӻ�����6�����ӣ���2�����Ӳ㣬��һ��2�����ӣ��ڶ���4�����ӣ�̼ԭ�ӵĽṹʾ��ͼΪ��
��̼ԭ�Ӻ�����6�����ӣ���2�����Ӳ㣬��һ��2�����ӣ��ڶ���4�����ӣ�̼ԭ�ӵĽṹʾ��ͼΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ��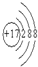 ��
�� ��
��

 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A�� |
B�� |
C�� |
D��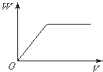 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Na+��H+��Cl-��NO3- |
| B��Na+��Mg2+��Cl-��SO42- |
| C��K+��Ba2+��OH-��I- |
| D��Cu2+��CO32-��Br-��ClO- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��pH=1����Һ�У�Na+��K+��C2O42-��CrO42- |
| B��ˮ�������c��H+��=1��10-13 mol/L����Һ�У�NH4+��Na+��SO42-��NO3- |
| C��c��OH- ��=0.1mol/L����Һ�У�Na+��CO32-��NO3-��AlO2- |
| D��0.1mol/LFeCl3��Һ�У�K+��NH4+��HCO3-��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
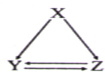
A������ԭ�ӽṹʾ��ͼ��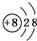 |
B��Na2O2�ĵ���ʽ��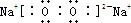 |
| C��HClO�Ľṹʽ��H-O-Cl |
| D��������Ϊ16�������ӣ�S2- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �±����и��������У�����ͨ��һ����Ӧʵ����ͼ��ʾת�����ǣ�������
�±����и��������У�����ͨ��һ����Ӧʵ����ͼ��ʾת�����ǣ�������| ѡ�� | X | Y | Z |
| A | AlCl3 | Al��OH��3 | NaAlO2 |
| B | C | CO | CO2 |
| C | CH2=CH2 | CH3CH2Br | CH3CH2OH |
| D | S | SO2 | SO3 |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com