
| y |
| 4 |
| 3mol |
| 6 |
| 12 |
| 4 |
 ��
�� ��
��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ݱ�����®�����Բ��ϲ�ҵ���š������Fe2O3���Ǵ��������� |
| B�����֣��Ϸʣ��������ý�̿������ʯΪԭ����������ԭ�����ý�ֱ̿�ӻ�ԭ����̬���� |
| C��Ϊ��֤��ij����ʯ�д��������ӣ��ɽ�����ʯ����������ټ�KSCN��Һ���� |
| D��ij����������ʯ�Ƶõ�FeS�ܳ�ȥ��ˮ�е�Hg2+���ɼ���ͬ�����£�Ksp��FeS����Ksp��HgS�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ʱ������������������ƽ�����̣��������������ƽ�������� |
| B������ʱ��Ӧʹ�¶ȼ�ˮ�������������ƿ�ڵ���Һ�� |
| C����Һʱ����Һ©���²�Һ����¿ڷų���Ȼ���ٽ��ϲ�Һ����¿ڷų� |
| D�������ᾧʱ��������е�ˮ�ֲ�����ȫ���ɣ�������ʹʣ��ˮ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����д�Ȼ�ѧ����ʽʱ�κη�Ӧ���������ܱ�ע |
| B���Ȼ�ѧ����ʽ�еĻ�ѧ��������ʾ���ʵ����������Ƿ��� |
| C���κ��������кͷ�Ӧ������1 mol H2Oʱ�ų������������к��� |
| D��101KPaʱ��1 mol H2�������� O2��ȼ�շų�����������H2��ȼ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
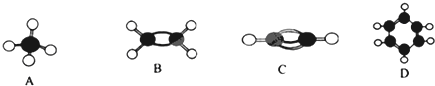
| A��A�Ķ���ȡ����ֻ��һ�֣�˵��AΪ����ṹ |
| B��B����ʹ���Ը��������Һ��ɫ |
| C��C�е�̼��������Ϊ6��1 |
| D��D��ʹ��ˮ��Ӧ����ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
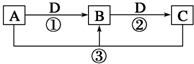 ��֪A��B��C��D����ѧ��ѧ�ij������ʣ���A��B��C������ͬһ��Ԫ�أ���һ������������֮����ת����ϵ��ͼ��ʾ�����ַ�Ӧ�е�H2O����ȥ����
��֪A��B��C��D����ѧ��ѧ�ij������ʣ���A��B��C������ͬһ��Ԫ�أ���һ������������֮����ת����ϵ��ͼ��ʾ�����ַ�Ӧ�е�H2O����ȥ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ��ʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��ͼ��ʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com