
����Ŀ�������Ѿ�ѧ���Ļ�ѧ֪ʶ���ش��������⡣
I.�������仯�������������������Ź㷺��Ӧ�ã�
(1)θ��ƽ(��Ҫ�ɷ�Ϊ��������)����������θ����࣬������������_______�ԣ�_______(��ܡ����ܡ�)������������Һ���档
(2)��Cu��ϡ�����ϣ����߲��ܷ�Ӧ������H2O2����Һ�ܿ�����ɫ���÷�Ӧ�Ļ�ѧ����ʽΪ____________________________________________��
(3)NaHSO4��һ����ʽ�Σ�д��NaHSO4��ˮ�еĵ��뷽��ʽ_________________________��
II.���ʵ�����ѧϰ��ѧ�Ļ�����
(1)14.4 g CO��CO2�Ļ�������ڱ�״������ռ�����Ϊ8.96 L������CO������Ϊ_____��
(2)����ŨH2SO4����������Ϊ98%���ܶ�Ϊ1.84g/cm3����Ũ��Ϊ________mol��L��1��
(3)19gij���۽������Ȼ���ACl2�к���0.4mo1Cl�����ӣ�����A�����ԭ��������_______��
(4)���ݷ�Ӧ14CuSO4��5FeS2��12H2O��7Cu2S��5FeSO4��12H2SO4����֪����2.5 mol FeS2�μӷ�Ӧʱ����������������ʵ���Ϊ_______mol��
���𰸡����� ���� Cu+H2SO4+H2O2=CuSO4+2H2O NaHSO4=Na++H++SO42- 5.6g 18.4 24 1.5
��������
I.��1��θ�����Ҫ�ɷ���HCl�������������������ԣ����к�θ�
��2��Cu��ϡ�����H2O2��ַ�Ӧ����������ͭ��ˮ��
��3��NaHSO4��һ����ʽ�Σ�Ϊǿ����ʣ���ȫ���룻
II.��1������������CO������Ϊxg��CO2������Ϊyg��������֪�����ж�Ԫһ�η����飬��һ�������CO��������
��2������c=![]() ����Ũ�����Ũ�ȣ�
����Ũ�����Ũ�ȣ�
��3������Cl�������ʵ��������ACl2�����ʵ���������n=![]() ����Ħ�����������õ�A�����ԭ��������
����Ħ�����������õ�A�����ԭ��������
��4����Ӧ��Cu��+2�۽��͵�+1�ۣ�S��-1�����ߵ�+6�ۣ����͵�-2�ۣ��ɴ˽��з����жϡ�
I.��1��θ�����Ҫ�ɷ���HCl�������������������ԣ��������Ƶļ��Թ�ǿ�����и�ʴ�ԣ�����к�θ��ʱ������������������Һ���棻
��2��Cu��ϡ�����H2O2��ַ�Ӧ����������ͭ��ˮ����Ӧ����ʽΪ��Cu+H2SO4+H2O2=CuSO4+2H2O��
��3��NaHSO4��һ����ʽ�Σ�Ϊǿ����ʣ���ȫ���룬���뷽��ʽΪ��NaHSO4=Na++H++SO42-��
II.��1������������CO������Ϊxg��CO2������Ϊyg��������֪�����ɵ�
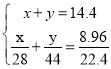 �����
�����![]() �����CO������Ϊ5.6g��
�����CO������Ϊ5.6g��
��2��c=![]() =
=![]() =18.4g/mol��
=18.4g/mol��
��3���Ȼ���ACl2�к���0.4mo1Cl������ôACl2�����ʵ���Ϊ0.2mo1��ACl2��Ħ������M=![]() =
=![]() =95g/mol�����A�����ԭ������Ϊ95-35.5��2=24��
=95g/mol�����A�����ԭ������Ϊ95-35.5��2=24��
��4����Ӧ��Cu��+2�۽��͵�+1�ۣ�S��-1�����ߵ�+6�ۣ����͵�-2�ۣ��ɵù�ϵʽ5FeS2~3S����˵���2.5mol FeS2�μӷ�Ӧʱ����������������ʵ���Ϊ2.5mol��![]() =1.5mol��
=1.5mol��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���йش����Ĵ�������������Դ����Ҵ�������ʵ�����õ�һЩ��ʶ��ij��ʦ�������ͼ��ʾװ��(�г�װ�õ���ʡ��)����ʵ�����Ϊ���Ȱ�ͼ��װ��װ�ã��رջ���a��b��c����ͭ˿���м䲿�ּ���Ƭ�̣�Ȼ�����a��b��c��ͨ�����ƻ���a��b�����н���(��Ъ��)��ͨ�����塣��M���۲쵽���Ե�ʵ�������Իش��������⣺
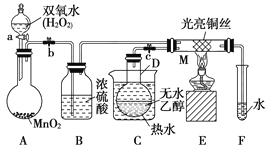
��1��д��A�з�����Ӧ�Ļ�ѧ����ʽ��_____________________��B��������____________________��C����ˮ��������_________________________________________��
��2��д��M��������Ӧ�Ļ�ѧ����ʽΪ_________________________��
��3����M���пɹ۲쵽������Ϊ_______________________�����п���ʶ����ʵ������д���________(�����μ����������μ���)��ѧ��Ӧ����������ʶ���������������Ҫһ����________��
��4��ʵ�����һ��ʱ�����������ƾ��ƣ���Ӧ________(������������������)�������У���ԭ����_________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
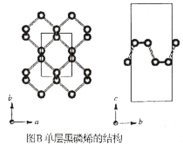
����Ŀ����������һ���ȶ���ͬ�������壬������������ϵ�ľ���ṹ��ͼA������������a=3.310A��b=4.380A��c=10.500A������ϩ�Ƕ�ά�ĵ�����ף�ͼB��������ϩ��ʯīϩ�ṹ���ƣ�P����λ��Ϊ3����ʯīϩ��ȣ�����ϩ���а뵼�����ʣ����ʺ�������������������֪���ṹ��ֻ��һ�ֵ�Ч������λP������Pԭ�ӵijɼ�����һ����ͼA�б��Ϊ�ٵ�Pԭ�ӵľ���������Ϊ��0.50��0.090��0.598������ش��������⣺
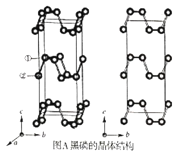
��1��д��Pԭ�ӵļ۵����Ų���___��
��2��P��F�ĵ縺�Դ�С˳����X(P)___X(F)��������<����=������>����P��F�γɵķ���PF3��PF5�����ǵļ��ι��ͷֱ�Ϊ__��__��
��3���ٺ�����Pԭ���ӻ�������__�������в�����__��ѡ����ĸ��ţ���
A.���ۼ� B.���� C.���� D.���»���
�ں��ס�����������۵�Ӹߵ��͵�˳��Ϊ__��ԭ����__��
��4��ͼA�б��Ϊ�ڵ�Pԭ�ӵľ���������Ϊ__�����ľ����к���__��Pԭ�ӡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������췼��̶����Ȫ������ϡ������Ԫ�ء�������ʯ�����������������п�������ܡ��̡�þ���Ƶ�30���ֶ������������Ԫ�ء�
�ش��������⣺
(1)��̬Geԭ�Ӽ۵����Ų�ͼΪ_______��Geԭ�ӵĵ��ӷ���ԾǨʱ�����ջ���ͬ�Ĺ⣬���ù������ǻ��______����(������������ ����״��)������GeԪ�صĴ��ڡ�
(2)����̼ͬ�壬���ʺͽṹ��һ���������ԣ���Ԫ�����γ���������(�������ƣ�Na2GeO3���������ƣ�Na2Ge2O5��)��Ҳ���γ�����������������(GenH2n+2)��
��Na2GeO3����ԭ�ӵ��ӻ���ʽ��______��
���Ʋ� 1molGenH2n+2�к��е���������Ŀ��_____(��NA��ʾ�����ӵ�����ֵ)��
(3)��������Һ��[EMIM] [AlCl4]�ɵ������ԭ����Ge�����۵�ֻ��7������ EMIM+�ṹ��ͼ��ʾ��
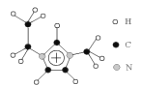
�ٸ����ʵľ���������_________��
��EMIM+ ���������Ԫ�صĵ縺����С�����˳����________��
(4)������NH3������H-N-H �ļ���Ϊ 107.3 ��[Zn(NH3)6]2+������H-N-H�ļ���________107.3�� ( ��������������С�������������� )��
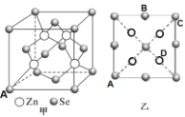
(5)����п( ZnSe)��һ����Ҫ�İ뵼����ϣ��侧���ṹ��ͼ����ʾ���þ�������ԭ�ӵ���λ����_____����֪�����߳�Ϊa pm����ͼΪ��ͼ�ĸ���ͼ��A������Ϊ( 0��0��0)��B������Ϊ(![]() ��a��
��a��![]() ) ���� D������Ϊ_______�����þ����ܶ�Ϊ�� g��cm-3�����ӵ�����NAΪ_______(�г�����ʽ)��
) ���� D������Ϊ_______�����þ����ܶ�Ϊ�� g��cm-3�����ӵ�����NAΪ_______(�г�����ʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ���ʾ����������صĻ�ѧ��Ӧ��ȫ��ȷ����
A.��FeCl3��Һ�в��ϼ�������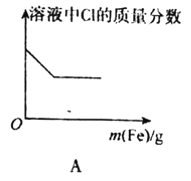
B.Fe(OH)2����¶���ڿ����������ı仯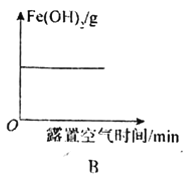
C.25��ʱ����Cl2ˮ��Һ��ͨ���������(�������ֳ�������) 
D.��AlCl3��Һ�в��ϵ����ռ���Һ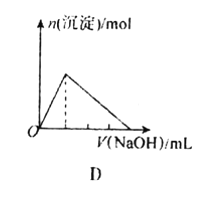
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�ʵ��˵������ȷ����
A.ij��Һ��ǿ����ȣ�����ʹʪ��ĺ�ɫʯ����ֽ���������壬�����Һ����Ϊ���
B.������������Ʒ����ϡ���ᣬ�μ����軯����Һ����Һ��ΪѪ��ɫ������֪����Ʒ�Ѿ���������
C.������FeBr2��FeI2�Ļ����Һ��ͨ��������������Ȼ�����Һ���ɣ��õ�FeCl3����
D.���з�̪��Na2CO3��Һ�м�������BaCl2���壬��Һ��ɫ��dz��֤��Na2CO3��Һ�д���ˮ��ƽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����άͪ���ˮ��Һ��һ�ֳ��õĵ���������������άͪͨ�������HI3�γɾ�άͪ�⣬��ṹ��ʾ��ͼ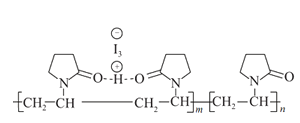 ������˵������ȷ���ǣ� ��
������˵������ȷ���ǣ� ��
A.��άͪ�ĵ�����![]() B.��άͪ������(m+n��������ۺ϶���
B.��άͪ������(m+n��������ۺ϶���
C.��άͪ����һ��ˮ��������D.��άͪ��һ���������ܷ���ˮ�ⷴӦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������һ��������Na2O���ʵ�Na2O2����������ͼ��ʾ��ʵ��װ�òⶨNa2O2�����Ĵ��ȡ�(�ɹ�ѡ�õ��Լ�ֻ��CaCO3���塢6 mol��L-1���ᡢ6 mol��L-1���������ˮ)�ش��������⣺
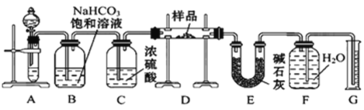
��1��װ��B��������___________________�� װ��E�м�ʯ�ҵ�������_________________________________��
��2��װ��D�з�����Ӧ�Ļ�ѧ����ʽ��_________________��______________________.
��3���������أ�KO2�����������һ��������CO2����̼���μ�O2��д���÷�Ӧ�Ļ�ѧ����ʽ_________________________��
��4������ʼʱ�����Ʒ������Ϊ2.0 g����Ӧ���������������Ϊ224 mL(��״��)����Na2O2�����Ĵ���Ϊ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ԫ��X��Y��Z��T��W�����ڱ��е�λ����ͼ��ʾ��Xԭ�����������������ڲ��������3��������˵����ȷ����
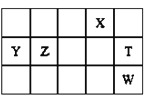
A.TԪ�صĵ�������ˮֻ��˷����Ӽ�������
B.TX2��T2��X3��������ɱ������
C.HT�����Ա�HW������ǿ
D.Y��X�γɼ��������������ͼӦ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com