
��ѧ����������Ĺؼ�����ѧΪ����������������ṩ�����ʱ�֤��
(1)���ʱ���öƲ������������������____________________________________��Ϊ��ʹ�Ʋ��Ⱦ��ȡ��⻬���ܡ���Ƽ��ĸ�����22____��
(2)±ˮ���̺��ŷḻ��þ��Դ����ת����ɻ��MgCl2�ֲ�Ʒ����±ˮ����ȡþ�IJ���Ϊ
a�������ߴ������ڵı������ճ�ʯ�ң�����ʯ���Ƴ�ʯ���飻
b����ʯ������뵽��ˮ�������о����˵õ�Mg(OH)2������
c����Mg(OH)2�����м�������õ�MgCl2��Һ���پ������ᾧ�õ�MgCl2·6H2O��
d����MgCl2·6H2O��һ�������¼��ȵõ���ˮMgCl2��
e��������ڵ��Ȼ�þ�ɵõ�Mg��
�ٲ���d�еġ�һ��������ָ����__________________��Ŀ����_________________��
��������ȡþ�������У�Ϊ�˽��ͳɱ���������Ⱦ�����Բ�ȡ�ܶ��ʩ����д������һ��________________________________________________________________________��
����ͬѧ��Ϊ������b��ɼ���Mg(OH)2�õ�MgO���ٵ�����ڵ�MgO�ƽ���þ�������ɼ�ʵ�鲽�裬��ͬ���ͬѧ���뷨��Ϊʲô��
(3)���Ǻ˷�Ӧ����Ҫ��ȼ�ϣ��Ѿ����Ƴɹ�һ���������ӽ�����֬����ר��������ˮ�е�U4����������������Ԫ�ء��䷴Ӧԭ��Ϊ____________________________________
________________________________________________________________________(��֬��HR����)��
�������ӽ���������ӽ���Ĥ���ᴦ�������������õ����˵���Һ���䷴Ӧԭ��Ϊ________________________________________________________________________
________________________________________________________________________��
(4)��˾ƥ��(COOHOOCCH3) �ڳ�ʪ�����пɷֽ��ˮ����ʹ�����Դ����ζ�����ܷⱣ�棬�û�ѧ����ʽ��ʾ��˾ƥ�ֱ����������ܱա����ﴦ��ԭ��______________
�ڳ�ʪ�����пɷֽ��ˮ����ʹ�����Դ����ζ�����ܷⱣ�棬�û�ѧ����ʽ��ʾ��˾ƥ�ֱ����������ܱա����ﴦ��ԭ��______________
________________________________________________________________________��
�˷�Ӧ����������________________________________________________________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������˪��As2O3����������п�������Ϸ�Ӧ�����ɵ��黯�⣨AsH3�����Ȳ���������ȫ�ֽ�ɵ���������������������Ϊ1.50mg����
A������������˪Ϊ1.98mg
B���ֽ����������Ϊ0.672ml
C������˪��Ӧ��пΪ3.90mg
D��ת�Ƶĵ�������Ϊ6��10��5NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ������ѧϰ��ѧ����Ҫ���ߣ����л�ѧ�����У���ȷ����
A����֪��2H2(g)��O2(g) �� 2H2O(g)����H����483.6 kJ/mol��������ȼ����Ϊ241.8 k J/mol
J/mol
B����AgCl����Һ�м���KI��Һ�����Ag+ + I- = AgI��
C��ij��Ӧ⊿H��0,��S>0,��÷�Ӧ�����������¾����Է����С�
D����ϡHNO3�ܽ�FeS���壺FeS+2H+=Fe2++H2S
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�������ȷ����
A������п�̵���У�MnO2 �Ǵ���
�Ǵ���
B����пŦ�۵�ع���ʱ��Ag2O����ԭΪAg
C���ŵ�ʱ��Ǧ������������Ũ�Ȳ�������
D�����ʱ�����ƵĽ�����Ʒ���淢����ԭ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ����
A���ϳɰ��ġ��������λ��������
B����Ƶ����Է�Һ�ü��кͺ�Ϳ����ŷ�
C����������Ĺ����У���Ϊ�������ϵ�����ú��������
D��ʹ��ú̿ת���Ĺܵ�ú����ֱ��ȼú�ɼ��ٻ�����Ⱦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ڹ�ҵ�� չ��ȼ���豸�������࣬�豸��ģ����������Щ�����ŷŵ������ж����д�����
չ��ȼ���豸�������࣬�豸��ģ����������Щ�����ŷŵ������ж����д����� SO2��������ͳ�ƣ��ҹ�1995�깤ҵSO2���ŷ���Ϊ1 396��֣�2006�깤ҵSO2���ŷ����ﵽ��3 800��֣�����SO2����Ⱦ���ҹ�ÿ����ʧ�ߴ�1 100��Ԫ��
SO2��������ͳ�ƣ��ҹ�1995�깤ҵSO2���ŷ���Ϊ1 396��֣�2006�깤ҵSO2���ŷ����ﵽ��3 800��֣�����SO2����Ⱦ���ҹ�ÿ����ʧ�ߴ�1 100��Ԫ��
(1)д��������ҵ���������в���SO2��ʵ����
��________________________________________________________________________��
��________________________________________________________________________��
(2)����SO2��Ⱦ�ɲ��õĴ�ʩ��(д������)��
��________________________________________________________________________��
��________________________________________________________________________��
��___________________ _____________________________________________________��
_____________________________________________________��
(3)ʪʽʯ��ʯ—ʯ�෨��������������������������һ�ַ������乤�������ǣ���������¯Ԥ�������������������������ַ�ú���̳����پ���һ��ר�ŵ��Ƚ�������Ȼ������������������е�SO2�뺬��ʯ��ʯ�Ľ�Һ������ Һ�Ӵ���ͨ�����������ʯ��(CaSO4·2H2O)���������������Ӧ��ѭ����������������ټ��ȣ������̴ѣ����������
Һ�Ӵ���ͨ�����������ʯ��(CaSO4·2H2O)���������������Ӧ��ѭ����������������ټ��ȣ������̴ѣ����������
��д��ʪ��ʯ��ʯ—ʯ�෨�������漰�Ļ�ѧ��Ӧ����ʽ��
________________________________________________________________________
________________________________________________________________________��
����ʯ��ʯ��Һ��SO2���ռ���������ʯ������SO2��ԭ���ǣ�________________________________________________________________________
_____________ ___________________________________________________________��
___________________________________________________________��
�����������еõ���ʯ�࣬������Ȼ�����(��Ҫ��Դ��ȼ��ú)�������ʼ���ֵ����ʯ���Ʒ���ܱ仵����ҵ�������������Ȼ���ķ�����__________________��
(4)ij��ѧ��ȤС��Ϊ�˲ⶨ������������ʯ������(CaSO4·xH2O)���ⶨxֵ��������ʵ�飺��ʯ�����ʹ֮��ˮ�����ȹ����й����������ʱ��ı仯��ϵ����ͼ��ʾ�����ݱ��������������Ϊ2.72 g���ٸı䡣��
��ʯ��Ļ�ѧʽ����ͼ����AB�ζ�Ӧ������Ļ�ѧʽ��
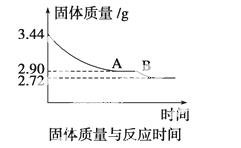
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ҵ�Դٽ����ú���ᷢչ������Ҫ���á�
(1)������¯��Ϊ�岿�֣�����ʯ�����ú����Ҫ��________���ַ�����Ӧ����________���ֿ�ʼ����������¯����
(2)����ʱ�����Ļ�ѧ����ʽΪ��_____________��
����衢�̺�����Ŀ����____________��
(3)����ֺ��е� Cr Ԫ���������ֹ��̵�����________(�ǰ����)���룬ԭ����_________________��
(4)���������������У�β�������е���Ҫ��Ⱦ����________���ӻ����;��ýǶȿ��ǣ�����β��������������_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij���������̿�(��MnO2Լ70%��Al2O3)����п��(��ZnSԼ80%��FeS)��ͬ����MnO2��Zn(�ɵ��ԭ��)��
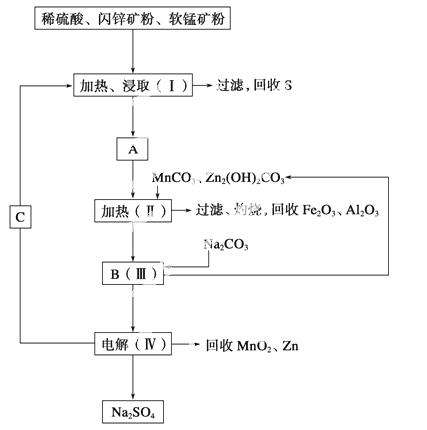
��֪����A��MnSO4��ZnSO4��Fe2(SO4)3��Al2(SO4)3�Ļ��Һ��
�ڢ��е�ⷽ��ʽΪMnSO4��ZnSO4��2H2O MnO2��Zn��2H2SO4��
MnO2��Zn��2H2SO4��
(1)A�����ڻ�ԭ�������______ ____��
____��
(2)����MnCO3��Zn2(OH)2CO3��������_____________________________________
________________________________________________________________________��
����Ҫ���ȵ�ԭ����____________________________________________________��
C�Ļ�ѧʽ��________________________��
(3)�������г��õ�MnO2��Zn���⣬���ɵõ��ĸ���Ʒ��________��
(4)��������������е���ģ�����ʯ�⣬�蹺��Ļ���ԭ����________��
(5)Ҫ��Na2SO4��Һ�еõ�â��(Na2SO4·10H2O)������еIJ���������Ũ����________�����ˡ�ϴ�ӡ�����ȡ�
(6)������MnO2��Zn�ĽǶȼ��㣬���̿����п��������ȴ�Լ��________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com