
| ��ѧʽ | ����ƽ�ⳣ�� |
| HCN | K=4.9��10-10 |
| CH3COOH | K=1.8��10-5 |
| H2CO3 | K1=4.4��10-7��K2=4.7��10-11 |
���� ��1����ĵ���ƽ�ⳣ��Խ�����������ˮ��̶�ԽС������ͬŨ�ȵ�������Һ����Խ����
��2��NaCN������CO2��Ӧ����NaHCO3��HCN��
��3����0.02mol/L��HCN��0.01mol/L ��NaOH��Һ�������ϣ���Һ�е������ǵ����ʵ���Ũ�ȵ�NaCN��HCN����֪�����Һ��c��CN-����c��Na+�������ݵ���غ��֪c��H+����c��OH-���������Һ�ʼ��ԣ�˵��CN-��ˮ��̶ȴ���HCN�ĵ���̶ȣ��ݴ˽����жϣ�
��4����������̼��������ӷ���˫ˮ�ⷴӦ���ɶ�����̼�������������������
��5�����ݲ��������Һ��ʾ���Լ�����غ��ж���Һ�и�����Ũ�ȴ�С��
��� �⣺��1������ƽ�ⳣ����CH3COOH��H2CO3��HCN����Ũ�ȵ�NaCN��Һ��Na2CO3��Һ��CH3COONa��Һˮ��̶�Ϊ��Na2CO3��Һ��NaCN��Һ��CH3COONa��Һ������Һ��pHΪ��Na2CO3��NaCN��CH3COONa��
�ʴ�Ϊ��b��a��c��
��2��NaCN������CO2��Ӧ����NaHCO3��HCN����Ӧ����ʽΪ��NaCN+CO2+H2O=NaHCO3+HCN��
�ʴ�Ϊ��NaCN+CO2+H2O=NaHCO3+HCN��
��3����0.02mol/L��HCN��0.01mol/L ��NaOH��Һ�������ϣ���Һ�е����������ʵ���Ũ�ȶ�Ϊ0.005mol•L-1��NaCN��HCN�����c��Na+����c��CN-�������ݵ���غ��֪��c��H+����c��OH-������Һ�ʼ��ԣ�����HCN��Ũ��Ϊ0.005mol•L-1��CN-��Ũ��С��0.005mol•L-1��
A�����ݷ�����֪����Һ�ʼ��ԣ�c��H+����c��OH-������A����
B�����Һ�ʼ��ԣ���c��H+����c��OH-������B��ȷ��
C������������Ũ�Ƚ�С��c��CN-��ԶԶ����c��OH-������C����
D�����������غ��֪��c��HCN��+c��CN-��=0.01mol/L����D��ȷ��
�ʴ�Ϊ��BD��
��4����������̼��������ӻ�Ϸ���˫ˮ�������������������Ͷ�����̼���壬��Ӧ�����ӷ���ʽΪ��Al3++3HCO3-�TAl��OH��3��+3CO2����
�ʴ�Ϊ��Al3++3HCO3-�TAl��OH��3��+3CO2����
��5������������Һ��ʾ���ԣ���HC2O4-�ĵ���̶ȴ�����ˮ��̶ȣ�����c��C2O42-����c��H2C2O4������������������ˮ�ĵ����HC2O4-�ĵ��룬��c��H+����c��C2O42-����HC2O4-��ˮ��̶Ƚ�С����c��HC2O4-����c��C2O42-����������Һ�и�����Ũ�ȴ�СΪ��c��Na+����c��HC2O4-����c��H+����c��C2O42-����c��OH-����
�ʴ�Ϊ��c��Na+����c��HC2O4-����c��H+����c��C2O42-����c��OH-����
���� ���⿼����������ʵĵ��뼰��Ӱ�졢����Ũ�ȴ�С�Ƚϡ�ԭ��ع���ԭ����Ӧ�õ�֪ʶ����Ŀ�Ѷ��еȣ���ȷ������ʵĵ���ƽ�⼰��Ӱ�����ء�ԭ��ع���ԭ��Ϊ���ؼ�������֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ�����ѧ���ķ������������������Ӧ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
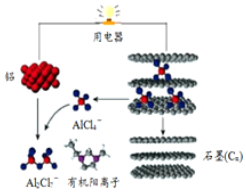 һ���ܿ��ٳ��ij������ܵ��-Ǧ���ӵ�أ������ܸ��õĽ�����������Ĵ洢��Э����ƽ�����⣮�õ����A1Cl${\;}_{4}^{-}$��A12Cl${\;}_{7}^{-}$i�Լ��л���������ɵ������Һ���ŵ�ʱ�Ĺ���ԭ����ͼ��ʾ�����й۵���ȷ���ǣ�������
һ���ܿ��ٳ��ij������ܵ��-Ǧ���ӵ�أ������ܸ��õĽ�����������Ĵ洢��Э����ƽ�����⣮�õ����A1Cl${\;}_{4}^{-}$��A12Cl${\;}_{7}^{-}$i�Լ��л���������ɵ������Һ���ŵ�ʱ�Ĺ���ԭ����ͼ��ʾ�����й۵���ȷ���ǣ�������| A�� | �õ�س��ʱ�ĵ�������Ϊ�������õ�����ʯī��C�� | |
| B�� | �õ�طŵ�ʱʯī�缫����������Ӧ | |
| C�� | ���ʱ��������ӦΪ��4Al2Cl7-+3e-�TAl+7AlCl4- | |
| D�� | �ŵ�ʱ���л������������缫�����ƶ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 3����ҺpH�Ĵ�С˳���� �ۣ��ڣ��� | |
| B�� | ����3����Һϡ����ͬ������pH�仯�����Ǣ� | |
| C�� | ���ֱ����25mL 0.1mol•L-1�����pH��С���Ǣ� | |
| D�� | �������¶ȣ���۵�pH���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ö��������������Һ�뽺�� | |
| B�� | ������ķ�����ȥ����ˮ�к��е�Cl- | |
| C�� | ����ȡ�ķ�����ijЩֲ������ȡ���ϻ���ҩ�ɷ� | |
| D�� | �����Ƴ��ڱ�¶�ڿ����е����ղ�����NaHCO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | c=$\frac{V��}{17V+22400}$ | B�� | ��=$\frac{17c}{100��}$ | ||
| C�� | ��=$\frac{17V}{17V+22400}$ | D�� | ��=$\frac{17V+22400}{22.4+22.4V}$ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��1���״���CH3OH����һ����Ҫ�Ļ���ԭ�ϣ���֪ CH3OH��1��+O2��g��=CO��g��+2H2O��g������H=-443.64kJ•mol-1
��1���״���CH3OH����һ����Ҫ�Ļ���ԭ�ϣ���֪ CH3OH��1��+O2��g��=CO��g��+2H2O��g������H=-443.64kJ•mol-1 2B+C��
2B+C���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Mg��Al�������õ�ⷨұ���õ� | |
| B�� | Na2O2��Na2O���Ԫ����ͬ����CO2��Ӧ����Ҳ��ͬ | |
| C�� | �ù�����KSCN��Һ��ȥFeCl2��Һ�е�����FeCl3 | |
| D�� | Na��Fe��һ����������ˮ��Ӧ������H2�Ͷ�Ӧ�ļ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʹ�ô������Լӿ췴Ӧ���� | |
| B�� | ����ѹǿ���Լӿ췴Ӧ���� | |
| C�� | ��Ӧ�ﵽƽ��ʱ��v������=v���棩 | |
| D�� | ����O2��������ʹNH3100%ת��ΪNO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ӵ�����v����v���������仯���ұ仯�ı������ | |
| B�� | ��ѹ��v����v����������v������v�� | |
| C�� | ���£�v����v����С����v��С��v�� | |
| D�� | ������䣬���뵪����v����v����������v������v�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com