
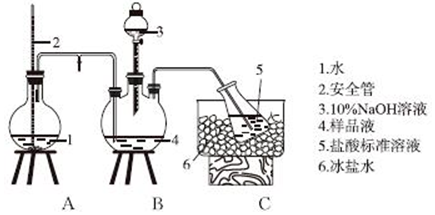
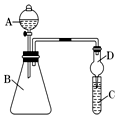
·ÖĪö £Ø1£©Éč·ØŹ¹×°ÖĆÄŚĶāŠĪ³ÉĘųŃ¹²īŹĒĘųĆÜŠŌ¼ģ²éµÄ³£ÓĆŹÖ¶Ī£¬¼ņŹö³ö¼ģ²é×°ÖĆĘųĆÜŠŌµÄÕāøö²Ł×÷¹ż³Ģ¼“æɽā“š£»
£Ø2£©ÓÉŅĒĘ÷½į¹¹ĢŲÕ÷£¬æÉÖŖŹ¢×°10%NaOHČÜŅŗµÄŅĒĘ÷Ćū³Ę£»
£Ø3£©øł¾Ż°±ĘųÓėŗĶ°±Ęų·“Ó¦ŃĪĖįÖ®¼äµÄ¹ŲĻµŹ½¼ĘĖć°±ĘųµÄÖŹĮ棬ŌŁøł¾ŻÖŹĮæ·ÖŹż¹«Ź½¼ĘĖć°±ÖŹĮæ·ÖŹż£»
£Ø4£©ĖįŠŌČÜŅŗÖ»ÄÜŹ¢·ÅŌŚĖįŹ½µĪ¶Ø¹ÜÖŠ£»øł¾Żn£ØCl-£©=c£ØAgNO3£©•V£ØAgNO3£©·ÖĪö²»µ±²Ł×÷¶ŌĻą¹ŲĪļĄķĮæµÄÓ°Ļģ£¬ŅŌ“ĖÅŠ¶ĻCl-µÄĮæµÄĪó²ī£»
£Ø5£©K2SĪŖÖøŹ¾¼Į£¬Ag2SĪŖשŗģÉ«£¬ÓƱź×¼ĻõĖįŅųµĪ¶Ø“ż²āŅŗ£¬µĪ¶ØÖÕµćµÄĻÖĻóŹĒµĪČė×īŗóŅ»µĪ±ź×¼ČÜŅŗ£¬Éś³ÉŗŚÉ«³Įµķ£¬ĒŅ30s ²»ø“Ō£»
£Ø6£©øł¾ŻĄė×Ó»ż³£ŹżŗĶc£ØAg+£©=1.0”Į10-5 mol•L-1¼ĘĖć$\frac{c£ØC{l}^{-}£©}{c£Ø{S}^{2-}£©}$£®
½ā“š ½ā£ŗ£Ø1£©ŹµŃéĒ°£¬°“Ķ¼×é×°ŗĆ×°ÖĆŗ󣬽«µ¼¹ÜÄ©¶ĖÉģČėĖ®ÖŠ£¬ÓĆŹÖĪę×”AÖŠµÄÉÕĘ棬Čōµ¼¹ÜæŚÓŠĘųÅŻĆ°³ö£¬ĒŅĖÉŹÖŗóµ¼¹ÜÄŚŠĪ³ÉŅ»¶ĪĖ®Öł£¬ŌņĘųĆÜŠŌĮ¼ŗĆ£®
¹Ź“š°øĪŖ£ŗŌŚĮ¬½ÓŗĆ×°ÖĆŗ󣬽«µ¼¹ÜŅ»¶ĖÉģČėĖ®ÖŠ£¬ÓĆŹÖĪę×”AÖŠµÄÉÕĘ棬Čōµ¼¹ÜæŚÓŠĘųÅŻĆ°³ö£¬ĒŅĖÉŹÖŗóµ¼¹ÜÄŚŠĪ³ÉŅ»¶ĪĖ®Öł£¬ŌņĘųĆÜŠŌĮ¼ŗĆ£»
£Ø2£©ÓÉŅĒĘ÷½į¹¹ĢŲÕ÷£¬æÉÖŖŹ¢×°10%NaOHČÜŅŗµÄŅĒĘ÷ĪŖ·ÖŅŗĀ©¶·£¬
¹Ź“š°øĪŖ£ŗ·ÖŅŗĀ©¶·£»
£Ø3£©Óė°±Ęų·“Ó¦µÄn£ØHCl£©=10-3V1L”Įc1mol•L-1-c2mol•L-1 ”Į10-3V2L=10-3£Øc1V1-c2V2£©mol£¬øł¾Ż°±ĘųŗĶHClµÄ¹ŲĻµŹ½ÖŖ£¬n£ØNH3£©=n£ØHCl£©=10-3£Øc1V1-c2V2£©mol£¬°±µÄÖŹĮæ·ÖŹż=$\frac{1{0}^{-3}£Ø{c}_{1}{V}_{1}{-c}_{2}{V}_{2}£©mol”Į17g/mol}{wg}$”Į100%£¬
¹Ź“š°øĪŖ£ŗ$\frac{1{0}^{-3}£Ø{c}_{1}{V}_{1}{-c}_{2}{V}_{2}£©mol”Į17g/mol}{wg}$£»
£Ø4£©ĖįŠŌČÜŅŗÖ»ÄÜŹ¢·ÅŌŚĖįŹ½µĪ¶Ø¹ÜÖŠ£¬ĖłŅŌŹ¢±ź×¼ČÜŅŗŃĪĖįµÄŅĒĘ÷ĪŖĖįŹ½µĪ¶Ø¹Ü£¬×°±ź×¼ŅŗµÄµĪ¶Ø¹ÜĪ“ÓƱź×¼ŅŗČóĻ“£¬ÅØ¶Č¼õŠ”£¬Ōģ³ÉV£ØAgNO3£©Ę«“ó£¬øł¾Żn£ØCl-£©=c£ØAgNO3£©•V£ØAgNO3£©·ÖĪö£¬æÉÖŖCl-µÄĮæĘ«“ó£¬
¹Ź“š°øĪŖ£ŗĖįŹ½µĪ¶Ø¹Ü£»Ę«“ó£»
£Ø5£©µĪ¶ØÖÕµćµÄĻÖĻóŹĒµĪČė×īŗóŅ»µĪĻõĖįŅųČÜŅŗŹ±£¬ČÜŅŗÖŠ³öĻÖŗŚÉ«øõĖįŅų³Įµķ£¬ĒŅ30s ²»ø“Ō£»
¹Ź“š°øĪŖ£ŗµĪČė×īŗóŅ»µĪ±ź×¼ČÜŅŗ£¬Éś³ÉŗŚÉ«³Įµķ£¬ĒŅ30s ²»ø“Ō£»
£Ø6£©c£ØCl-£©=$\frac{Ksp}{c£ØA{g}^{+}£©}$=$\frac{1.8”Į1{0}^{-10}}{1.0”Į1{0}^{-5}}$=1.8”Į10-5£¬c£ØS2-£©=$\frac{Ksp}{{c}^{2}£ØA{g}^{+}£©}$=$\frac{6.3”Į1{0}^{-50}}{£Ø1.0”Į1{0}^{-5}£©^{2}}$=6.3”Į10-40£¬$\frac{c£ØC{l}^{-}£©}{c£Ø{S}^{2-}£©}$=$\frac{1.8”Į1{0}^{-5}}{6.3”Į1{0}^{-40}}$=2.86”Į1034£¬
¹Ź“š°øĪŖ£ŗ2.86”Į1034
µćĘĄ ±¾Ģāæ¼²éĮĖĪļÖŹŗ¬ĮæµÄ²ā¶Ø£¬Éę¼°ÄŃČÜĪļµÄČܽāĘ½ŗā”¢Ńõ»Æ»¹Ō·“Ó¦”¢ĪļÖŹŗ¬ĮæµÄ²ā¶ØµČÖŖŹ¶µć£¬Ć÷Č·ŹµŃéŌĄķŹĒ½ā±¾Ģā¹Ų¼ü£¬ÖŖµĄÖøŹ¾¼ĮµÄєȔ·½·Ø£¬¼ģ²é×°ÖƵÄĘųĆÜŠŌŹĒŹµŃéŹŅÖĘČ”ĘųĢåµÄÖ÷ŅŖ²½Öč£¬Ó¦øĆŹģĮ·ÕĘĪÕ£»ĢāÄæÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
 X”¢Y”¢ZĪŖŌ×ÓŠņŹżŅĄ“ĪŌö“óµÄ¶ĢÖÜĘŚÖ÷×åŌŖĖŲ£¬ČżÖÖŌŖĖŲŹōÓŚ²»Ķ¬ÖÜĘŚ£¬ĻĀĮŠ×Ŗ»Æ¹ŲĻµÖŠ£¬A”¢B”¢CŹĒX”¢Y”¢Z¶ŌÓ¦µÄČżÖÖĘųĢ¬µ„ÖŹ£¬ĘäÓą¾łĪŖ³£¼ū»ÆŗĻĪļ£¬ĻĀĮŠ·ÖĪöÕżČ·µÄŹĒ£Ø””””£©
X”¢Y”¢ZĪŖŌ×ÓŠņŹżŅĄ“ĪŌö“óµÄ¶ĢÖÜĘŚÖ÷×åŌŖĖŲ£¬ČżÖÖŌŖĖŲŹōÓŚ²»Ķ¬ÖÜĘŚ£¬ĻĀĮŠ×Ŗ»Æ¹ŲĻµÖŠ£¬A”¢B”¢CŹĒX”¢Y”¢Z¶ŌÓ¦µÄČżÖÖĘųĢ¬µ„ÖŹ£¬ĘäÓą¾łĪŖ³£¼ū»ÆŗĻĪļ£¬ĻĀĮŠ·ÖĪöÕżČ·µÄŹĒ£Ø””””£©| A£® | Ąė×Ó°ė¾¶£ŗY£¾Z | B£® | ZµÄŗ¬ŃõĖį¾łĪŖĒæĖį | ||
| C£® | ÓėYĶ¬ÖÜĘŚĒč»ÆĪļÖŠD×īĪČ¶Ø | D£® | Fŗ¬Ąė×Ó¼üŗĶ¹²¼Ū¼ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 MnSO4ŌŚ¹¤ŅµÖŠÓŠÖŲŅŖÓ¦ÓĆ£®ÓĆČķĆĢæ󽬣ØÖ÷ŅŖ³É·ÖĪŖMnO2ŗĶĖ®£¬ŗ¬ÓŠFe2O3”¢FeO”¢Al2O3ŗĶÉŁĮæPbOµČŌÓÖŹ£©½ž³öÖʱøMnSO4£¬Ęä¹ż³ĢČēĻĀ£ŗ
MnSO4ŌŚ¹¤ŅµÖŠÓŠÖŲŅŖÓ¦ÓĆ£®ÓĆČķĆĢæ󽬣ØÖ÷ŅŖ³É·ÖĪŖMnO2ŗĶĖ®£¬ŗ¬ÓŠFe2O3”¢FeO”¢Al2O3ŗĶÉŁĮæPbOµČŌÓÖŹ£©½ž³öÖʱøMnSO4£¬Ęä¹ż³ĢČēĻĀ£ŗ| Ąė×Ó | Fe2+ | Fe3+ | Al3+ | Mn2+ | Pb2+ |
| æŖŹ¼³ĮµķŹ±µÄpH | 7.6 | 2.7 | 3.8 | 8.3 | 8.0 |
| ĶźČ«³ĮµķŹ±µÄpH | 9.7 | 3.7 | 4.7 | 9.8 | 8.8 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
| ĪļÖŹ | Al | Al2O3 | Fe | Fe2O3 |
| ČŪµć/”ę | 660 | 2 054 | 1 535 | 1 462 |
| ·Šµć/”ę | 2 467 | 2 980 | 2 750 | - |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
 Ä³Ń§Ļ°Š”×éĶ¬Ń§ĄūÓĆČēĶ¼×°ÖĆĄ“ŃéÖ¤Ķ¬Ö÷×åŌŖĖŲ·Ē½šŹōŠŌµÄ±ä»Æ¹ęĀÉ£ŗ
Ä³Ń§Ļ°Š”×éĶ¬Ń§ĄūÓĆČēĶ¼×°ÖĆĄ“ŃéÖ¤Ķ¬Ö÷×åŌŖĖŲ·Ē½šŹōŠŌµÄ±ä»Æ¹ęĀÉ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ńõ»ÆŠŌ£ŗCl2£¾ŗģĮ× | B£® | µ„ÖŹ·Šµć£ŗŗģĮ×£¾C12 | ||
| C£® | Ēā»ÆĪļĪČ¶ØŠŌ£ŗHC1£¾PH3 | D£® | ĖįŠŌ£ŗHClO4£¾H3PO4 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com