

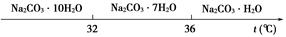
| ���� | CaCO3 | MgCO3 | Ca(OH)2 | Mg(OH)2 | Fe(OH)3 |
| Ksp | 4.96��10��9 | 6.82��10��6 | 4.68��10��6 | 5.61��10��12 | 2.64��10��39 |
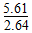 ��1021��2.125��1021
��1021��2.125��1021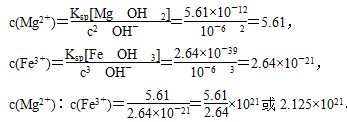



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������л������ۣ���������ռ���Һ��ַ�Ӧ����� |
| B��FeCl2��Һ�л���FeCl3������������۳�ַ�Ӧ����� |
| C��Na2CO3�����л�������NaHCO3���������NaOH��Һ����Ӧ��������� |
| D�������л��������Ȼ������壺���������ͨ��ʢ����ʳ��ˮ��ϴ��ƿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
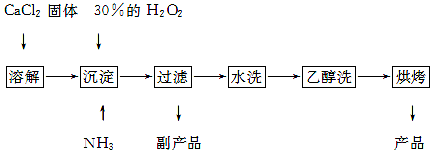
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
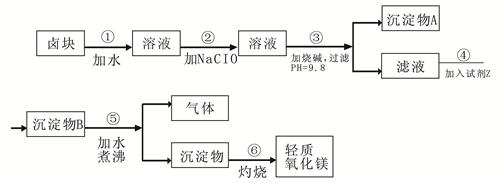
| ���� | ��ʼ���� | ������ȫ |
| Fe��OH��3 | 2.7 | 3.7 |
| Fe��OH��2 | 7.6 | 9.6 |
| Mn��OH��2 | 8.3 | 9.8 |
| Mg��OH��2 | 9.6 | 11.1 |
| �Լ� | �۸�Ԫ/�֣� |
| ƯҺ����NaClO��25.2%�� | 450 |
| ˫��ˮ����H2O2 ,30%�� | 2400 |
| �ռ��98% NaOH�� | 2100 |
| �����99.5% Na2CO3�� | 600 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
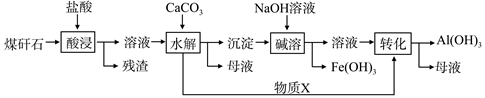
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ʵ����� | ���� | ���ͻ���� |
| A | ����ҺX���ȵμ�ϡ���ᣬ�ٵμ�Ba(NO3)2��Һ | ���ְ�ɫ���� | ��ҺX��һ������SO42�� |
| B | �ò�˿պȡ��ҺY������ɫ��Ӧ | ����ʻ�ɫ | ��ҺY�к�Na��������K�� |
| C | ��һ��Ũ�ȵ�Na2SiO3��Һ��ͨ������CO2���� | ���ְ�ɫ���� | H2SiO3�����Ա�H2CO3������ǿ |
| D | ��Na2CO3��Һ��ͨ������CO2 | ��Һ����� | ������NaHCO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com