
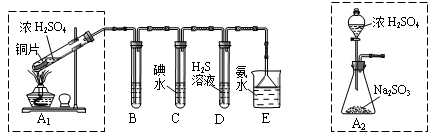
| ŹµŃé²Ł×÷ | Ō¤ĘŚĻÖĻóÓė½įĀŪ |
| ²½Öč1£ŗČ”ŹŹĮæ”°ĪüŹÕŅŗ”±ÓŚŠ”ÉÕ±ÖŠ£¬ÓĆ½ŗĶ·µĪ¹ÜČ”l mol/L BaCl2ČÜŅŗĻņŠ”ÉÕ±µĪ¼ÓÖ±ÖĮ¹żĮ攣 | Čō³öĻÖ°×É«»ė×Ē£¬ŌņČÜŅŗÖŠ“ęŌŚSO32-»ņ SO42-”£ |
| ²½Öč2£ŗ½«Š”ÉÕ±ÖŠµÄ×ĒŅŗ¹żĀĖ”¢Ļ“µÓ£¬ŌŁÓĆŹŹĮæĖ®°Ńø½ŌŚĀĖÖ½ÉĻµÄ¹ĢĢå³åČėĮķŅ»Š”ÉÕ±ÖŠ£»Ļņ³åĻĀµÄ¹ĢĢå ”£ | ”£ |
| ²½Öč3£ŗ ”£ | ”£ |
| ŹµŃé²Ł×÷ | Ō¤ĘŚĻÖĻóÓė½įĀŪ |
| ²½Öč2£ŗµĪČė1µĪ£Ø»ņÉŁĮæ£©Ę·ŗģČÜŅŗ£¬ŌŁµĪČė¹żĮæ2mol/LŃĪĖį£¬Õńµ“ | ČōĘ·ŗģĶŹÉ«£Ø»ņÓŠĘųÅŻ£©£¬Ōņ”°ĪüŹÕŅŗ”±ÖŠ“ęŌŚ SO32-”£ |
| ²½Öč3£ŗÓĆŹŌ¹ÜČ”ŹŹĮæĀĖŅŗ£¬ĻņĘäÖŠµĪČė¹żĮæµÄ1mol/LBa(OH)2ČÜŅŗ [»ņµĪČė1µĪ£Ø»ņÉŁĮæ£©Ę·ŗģČÜŅŗ£¬ŌŁµĪČė2-3µĪ£Ø»ņ¹żĮ棩µÄ2mol/LŃĪĖį]£¬Õńµ“”£ | Čō³öĻÖ°×É«³Įµķ£Ø»ņĘ·ŗģČÜŅŗĶŹÉ«£¬»ņÓŠĘųÅŻ£¬£©£¬Ōņ”°ĪüŹÕŅŗ”±ÖŠ“ęŌŚ HSO3-”£ |


 ×ß½ųĪÄŃŌĪÄĻµĮŠ“š°ø
×ß½ųĪÄŃŌĪÄĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¼ÓČėĶµÄÖŹĮæĪŖ6.4g |
| B£®¼ÓČėÅØĮņĖįÖŠČÜÖŹ0.2mol |
| C£®¼ÓČėĶµÄÖŹĮæ“óÓŚ6.4g |
| D£®¼ÓČėÅØĮņĖįÖŠŗ¬ČÜÖŹ¶ąÓŚ0.2mol |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Įņ»ÆĒāĶØČėÅØH2SO4ÖŠ | B£®Ļ”H2SO4ŗĶŅŅĖįŅŅõ„¹²ČČ |
| C£®ÅØH2SO4ŗĶC2H5OH¹²Čȵ½170”ę | D£®³±ŹŖµÄĀČĘųĶعżŹ¢ÓŠÅØH2SO4µÄĻ“ĘųĘæ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗ¼ĘĖćĢā
| ŹµŃé×鱚 | ¢Ł | ¢Ś | ¢Ū | ¢Ü |
| ĮņĖįČÜŅŗµÄĢå»ż£ØmL£© | 50 | 50 | 50 | 50 |
| ŃĪµÄÖŹĮæ£Øg£© | 9.260 | 13.890 | 20.835 | 32.410 |
| ¶žŃõ»ÆĮņµÄĢå»ż£ØmL£© | 1344 | 2016 | 3024 | 2464 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®“ņĄ×Ź±Ņ²ÄܲśÉś³ōŃõ£¬³ōŃõ·Ö×ÓŹĒÖ±ĻߊĶ·Ö×Ó |
| B£®³ōŃõ×Ŗ»ÆĪŖŃõĘųŗĶŃõĘų×Ŗ»ÆĪŖ³ōŃõ¾łŠėĪüŹÕÄÜĮæ |
| C£®³ōŃõŗĶŃõĘųµÄĻą»„×Ŗ»ÆÄܱ£³Ö“óĘųÖŠ³ōŃõµÄŗ¬Įæ»ł±¾ĪČ¶Ø |
| D£®Ļņ“óĘųÖŠÅŷŵŖµÄŃõ»ÆĪļŗĶ·śĀČ“śĢž¾łÄܼÓæģ³ōŃõµÄ·Ö½ā |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
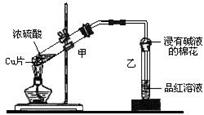
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
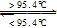 S£Øµ„Š±£¬¹Ģ£©”÷H=+0.398kJ”¤mol£1ČōNAĪŖ°¢·ü¼ÓµĀĀŽ³£Źż£¬ŌņĻĀĮŠĖµ·ØÖŠ£¬“ķĪóµÄŹĒ£Ø £©
S£Øµ„Š±£¬¹Ģ£©”÷H=+0.398kJ”¤mol£1ČōNAĪŖ°¢·ü¼ÓµĀĀŽ³£Źż£¬ŌņĻĀĮŠĖµ·ØÖŠ£¬“ķĪóµÄŹĒ£Ø £©| A£®³£ĪĀĻĀŠ±·½Įņ±Čµ„Š±ĮņĪČ¶Ø |
| B£®µ„Š±ĮņŗĶŠ±·½ĮņÖ®¼äµÄ×Ŗ»ÆŹōÓŚĪļĄķ±ä»Æ |
| C£®µ„Š±ĮņŗĶŠ±·½ĮņŌŚ³ä×ćµÄŃõĘųÖŠČ¼ÉÕ¾łÉś³ÉSO2 ? |
| D£®64 g µ„Š±ĮņŗĶŠ±·½ĮņµÄ»ģŗĻĪļŗ¬ĮņŌ×ÓŹżÄæĪŖ2NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®²£Į§ | B£®ĀĮ | C£®Ģś | D£®Ķ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ŌŚÕįĢĒÖŠ¼ÓČėÅØH2SO4ŗó³öĻÖ·¢ŗŚĻÖĻó£¬ĖµĆ÷ÅØH2SO4¾ßÓŠĶŃĖ®ŠŌ |
| B£®ÅØH2SO4ŗĶCu¼ÓČČ·“Ó¦£¬±ķĻÖÅØH2SO4µÄĒæŃõ»ÆŠŌŗĶĖįŠŌ |
| C£®³£ĪĀĻĀ£¬ÅØĮņĖįæÉŅŌÓĆĀĮ¹ŽÖü“ę£¬ĖµĆ÷ĀĮÓėÅØH2SO4²»·“Ó¦ |
| D£®Ą¶É«ĮņĖįĶ¾§Ģå¼ÓČėÅØH2SO4ŗó±ä°×£¬ĖµĆ÷ÅØH2SO4¾ßÓŠĪüĖ®ŠŌ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com