
 -COOCH2CH3��A���٢ڷ�Ӧ��C��D��E��B���٢ڲ���Ӧ��E��F��H������Ӧ���̣��������ʼ����ϵ��ͼ��
-COOCH2CH3��A���٢ڷ�Ӧ��C��D��E��B���٢ڲ���Ӧ��E��F��H������Ӧ���̣��������ʼ����ϵ��ͼ��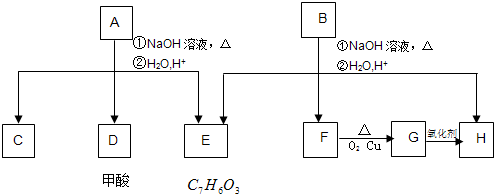
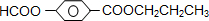
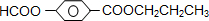

| Ũ���� |
| �� |
| Ũ���� |
| �� |
 -COOH�����F��G��H����֪FΪCH3CH2OH��GΪCH3CHO��HΪCH3COOH�������廯����A��B��Ϊͬ���칹�壬A��ˮ�����ɼ��ᡢHO-
-COOH�����F��G��H����֪FΪCH3CH2OH��GΪCH3CHO��HΪCH3COOH�������廯����A��B��Ϊͬ���칹�壬A��ˮ�����ɼ��ᡢHO- -COOH����C�ķ���ʽӦΪC3H80������ΪCH3CH2CH2OH��CH3CHOHCH3���Դ˿�ȷ��A�Ŀ��ܽṹΪ
-COOH����C�ķ���ʽӦΪC3H80������ΪCH3CH2CH2OH��CH3CHOHCH3���Դ˿�ȷ��A�Ŀ��ܽṹΪ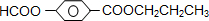 ��
�� ������л���Ľṹ�������Լ���Ŀ��Ҫ��ɽ����⣮
������л���Ľṹ�������Լ���Ŀ��Ҫ��ɽ����⣮ -COOH�����F��G��H����֪FΪCH3CH2OH��GΪCH3CHO��HΪCH3COOH�������廯����A��B��Ϊͬ���칹�壬A��ˮ�����ɼ��ᡢHO-
-COOH�����F��G��H����֪FΪCH3CH2OH��GΪCH3CHO��HΪCH3COOH�������廯����A��B��Ϊͬ���칹�壬A��ˮ�����ɼ��ᡢHO- -COOH����C�ķ���ʽӦΪC3H80������ΪCH3CH2CH2OH��CH3CHOHCH3���Դ˿�ȷ��A�Ŀ��ܽṹΪ
-COOH����C�ķ���ʽӦΪC3H80������ΪCH3CH2CH2OH��CH3CHOHCH3���Դ˿�ȷ��A�Ŀ��ܽṹΪ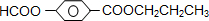 ��
�� ��
�� -COOH�����еĹ�����Ϊ�ǻ����Ȼ����ʴ�Ϊ���ǻ����Ȼ���
-COOH�����еĹ�����Ϊ�ǻ����Ȼ����ʴ�Ϊ���ǻ����Ȼ���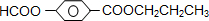 ��
�� ��
��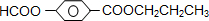 ��
�� ��
��| Ũ���� |
| �� |
| Ũ���� |
| �� |


 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
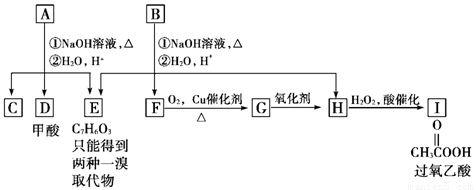
(12��)��ѧʽΪC11H12O4�ķ����廯����A��B��Ϊͬ���칹�壬A��D���ܷ���������Ӧ��E��һ��ȡ����ͬ���칹�������֣�A���٢�������Ӧ��C��D��E��B���٢�������Ӧ��E��F��H��������Ӧ���̡��������ʼ����ϵ��ͼ��ʾ��
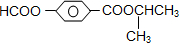
(1)A��2�ֿ��ܽṹ��д����ṹ��ʽ��____________________________________��
(2)��B��C��D��F��G��I�������У���Ϊͬϵ�����__________________________��
(3)д��D��F�ڼ��Ⱥ�ŨH2SO4�������·�����Ӧ�Ļ�ѧ����ʽ��
_________________________________����Ӧ���ͣ�__________________��
(4)��������Ӧ�͵�ȼ������ѡ����������ʵ�鷽��֤��D���л�ԭ�ԣ��밴Ҫ����д�±���
|
| ��ѡ�Լ��Ļ�ѧʽ | �۲쵽��ʵ������ |
| ����1 |
|
|
| ����2 |
|
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��7�֣�ÿ��1�֣������廯����A��B��Ϊͬ���칹�壬B�Ľṹ��ʽ��
��A���٢ڷ�Ӧ��C��D��E��B���٢ڷ�
Ӧ��E��F��H��������Ӧ���̣��������ʼ����ϵ��ͼ��
��1��E�к��й����ŵ�������__________________��
��2��A�����ֿ��ܵĽṹ����ṹ��ʽ�ֱ�Ϊ_____________��______________��
��3��C��D��F��G��H�������л�Ϊͬϵ�����___________��__________��
��4��C��Ũ�����¼��ȷ�����Ӧ�������ﲻʹ��ˮ��ɫ���������ʵĽṹ��ʽΪ��______________________________��
��5��д����G����������F�ķ�Ӧ����_________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��̫ԭ���и߶���ѧ�����п������ƻ�ѧ�Ծ��������棩 ���ͣ������
(12��)��ѧʽΪC11H12O4�ķ����廯����A��B��Ϊͬ���칹�壬A��D���ܷ���������Ӧ��E��һ��ȡ����ͬ���칹�������֣�A���٢�������Ӧ��C��D��E��B���٢�������Ӧ��E��F��H��������Ӧ���̡��������ʼ����ϵ��ͼ��ʾ��
(1)A��2�ֿ��ܽṹ��д����ṹ��ʽ��____________________________________��
(2)��B��C��D��F��G��I�������У���Ϊͬϵ�����__________________________��
(3)д��D��F�ڼ��Ⱥ�ŨH2SO4�������·�����Ӧ�Ļ�ѧ����ʽ��
_________________________________����Ӧ���ͣ�__________________��
(4)��������Ӧ�͵�ȼ������ѡ����������ʵ�鷽��֤��D���л�ԭ�ԣ��밴Ҫ����д�±���
|
|
��ѡ�Լ��Ļ�ѧʽ |
�۲쵽��ʵ������ |
|
����1 |
|
|
|
����2 |
|
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�߶���ѧ�����п��Ի�ѧ���� ���ͣ������
��7�֣�ÿ��1�֣������廯����A��B��Ϊͬ���칹�壬B�Ľṹ��ʽ��
 ��A���٢ڷ�Ӧ��C��D��E��B���٢ڷ�
��A���٢ڷ�Ӧ��C��D��E��B���٢ڷ�
Ӧ��E��F��H��������Ӧ���̣��������ʼ����ϵ��ͼ��

��1��E�к��й����ŵ�������__________________��
��2��A�����ֿ��ܵĽṹ����ṹ��ʽ�ֱ�Ϊ_____________��______________��
��3��C��D��F��G��H�������л�Ϊͬϵ�����___________��__________��
��4��C��Ũ �����¼��ȷ�����Ӧ�������ﲻʹ��ˮ��ɫ���������ʵĽṹ��ʽΪ��______________________________��
�����¼��ȷ�����Ӧ�������ﲻʹ��ˮ��ɫ���������ʵĽṹ��ʽΪ��______________________________��
��5��д����G����������F�ķ�Ӧ����_________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com