½šŹōÄĘ”¢øʵČŌŚŃõĘųÖŠČ¼ÉÕæÉÉś³É¹żŃõ»ÆĪļ£®“Ó×é³ÉÉĻ·ÖĪö£¬¹żŃõ»ÆĪļĪŖ½šŹōŃõ»ÆĪļ£¬æÉÄÜ»įÓėĖ®”¢¶žŃõ»ÆĢ¼”¢¶žŃõ»ÆĮņ·“Ӧɜ³ÉŃĪ£®
£Ø1£©Č”Ņ»Ö§Š”ŹŌ¹Ü£¬ĻņĘäÖŠ¼ÓČė¹żŃõ»ÆÄɹĢĢå£¬Č»ŗó¼ÓČėÉŁĮæÕōĮóĖ®£¬½«“ųÓą½żµÄŠ”ľĢõ²åČėŹŌ¹ÜÖŠ£¬¹Ū²ģµ½µÄĻÖĻóŹĒ
ÓŠĘųĢå·Å³ö£¬Š”ľĢõø“Č¼
ÓŠĘųĢå·Å³ö£¬Š”ľĢõø“Č¼
£¬·“Ó¦ŗó£¬ĻņĘäÖŠµĪČė·ÓĢŖČÜŅŗ£¬¹Ū²ģµ½µÄĻÖĻóŹĒ
ČÜŅŗ±äŗģ
ČÜŅŗ±äŗģ
£®øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
2Na2O2+2H2O=4NaOH+O2ӟ
2Na2O2+2H2O=4NaOH+O2ӟ
£®
£Ø2£©ÓŠĮ½øöŹµŃ銔×éµÄĶ¬Ń§ÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆ½ųŠŠŹµŃ飬Ą“Ģ½¾æ¹żŃõ»ÆÄĘÓė¶žŃõ»ÆĮņµÄ·“Ó¦
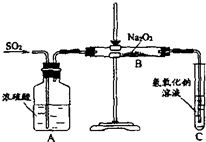
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
¢Ł×°ÖĆCÖŠĒāŃõ»ÆÄĘČÜŅŗµÄ×÷ÓĆŹĒ
ĪüŹÕ¶žŃõ»ÆĮņĘųĢå
ĪüŹÕ¶žŃõ»ÆĮņĘųĢå
¢Ś¼××éĶ¬Ń§ČĻĪŖNa
2O
2ÓėSO
2·“Ӧɜ³ÉĮĖNa
2SO
3ŗĶO
2£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ£ŗ
2Na2O2+2SO2=4Na2SO3+O2ӟ
2Na2O2+2SO2=4Na2SO3+O2ӟ
ĒėÉč¼ĘŅ»ÖÖŹµŃé·½°øÖ¤Ć÷Na
2O
2ÓėSO
2·“Ӧɜ³ÉµÄ°×É«¹ĢĢåÖŠŗ¬ÓŠNa
2SO
3£®
Č”°×É«¹ĢĢ壬¼ÓČėĻ”ĮņĖį£¬²śÉśÄÜŹ¹Ę·ŗģČÜŅŗĶŹÉ«µÄĘųĢå
Č”°×É«¹ĢĢ壬¼ÓČėĻ”ĮņĖį£¬²śÉśÄÜŹ¹Ę·ŗģČÜŅŗĶŹÉ«µÄĘųĢå
¢ŪŅŅ×éĶ¬Ń§ČĻĪŖNa
2O
2ÓėSO
2·“Ó¦³żĮĖÉś³ÉNa
2SO
3ŗĶO
2Ķā£¬»¹ÓŠNa
2SO
4Éś³É£®ĪŖ¼ģŃéŹĒ·ńÓŠNa
2SO
4Éś³É£¬ĖūĆĒÉč¼ĘĮĖČēĻĀ·½°ø£ŗ
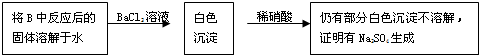
ÉĻŹö·½°øŹĒ·ńŗĻĄķ£æ
²»ŗĻĄķ
²»ŗĻĄķ
£®Ēė¼ņŅŖĖµĆ÷Į½µćĄķÓÉ£ŗ¢Ł
Ļ”ĻõĖįÄÜŹ¹ŃĒĮņĖį±µ×Ŗ»ÆĪŖĮņĖį±µ
Ļ”ĻõĖįÄÜŹ¹ŃĒĮņĖį±µ×Ŗ»ÆĪŖĮņĖį±µ
£»¢Ś
Čō·“Ó¦ŗó²ŠĮō¹żŃõ»ÆÄĘ£¬ĖüČÜÓŚĖ®ŗóÄܽ«SO32-×Ŗ»ÆĪŖSO42-
Čō·“Ó¦ŗó²ŠĮō¹żŃõ»ÆÄĘ£¬ĖüČÜÓŚĖ®ŗóÄܽ«SO32-×Ŗ»ÆĪŖSO42-
£®
£Ø3£©ĪŖĮĖ½ā¾öŃų½šÓćµÄĪüŃõĪŹĢā£¬æÉŌŚĖ®ÖŠ¼ÓČė¹żŃõ»ÆøĘ£®Ä³¹żŃõ»ÆÄĘѳʷ2.0g£¬¼ÓČėµ½×ćĮæµÄĖ®ÖŠ£¬ŌŚ±ź×¼×“æöĻĀÉś³ÉĮĖ224mLŃõĘų£®ŹŌ¼ĘĖćøĆѳʷ֊ŗ¬¹żŃõ»ÆøʵÄÖŹĮæ·ÖŹż£®

 ÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆ½ųŠŠµē½ā£®ĶصēŅ»»į¶ł£¬·¢ĻÖŹŖČóµÄµķ·ŪKIŹŌÖ½µÄC¶Ė±äĪŖĄ¶É«£®
ÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆ½ųŠŠµē½ā£®ĶصēŅ»»į¶ł£¬·¢ĻÖŹŖČóµÄµķ·ŪKIŹŌÖ½µÄC¶Ė±äĪŖĄ¶É«£®

 æŚĖćŠÄĖćĖŁĖćÓ¦ÓĆĢāĻµĮŠ“š°ø
æŚĖćŠÄĖćĖŁĖćÓ¦ÓĆĢāĻµĮŠ“š°ø Ķ¬²½ĶŲÕ¹ŌĶĮĻµĮŠ“š°ø
Ķ¬²½ĶŲÕ¹ŌĶĮĻµĮŠ“š°ø
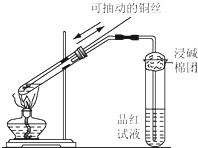 ijĶ¬Ń§ÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆ½ųŠŠĶÓėÅØĮņĖį·“Ó¦µÄŹµŃ飮Ēė»Ų“šĪŹĢā£®
ijĶ¬Ń§ÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆ½ųŠŠĶÓėÅØĮņĖį·“Ó¦µÄŹµŃ飮Ēė»Ų“šĪŹĢā£®

 ijæĪĶā»ī¶ÆŠ”×é×¼±øÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆ½ųŠŠŹµŃ飮ĻÖÓŠ¼×”¢ŅŅ”¢±ūČżĪ»Ķ¬Ń§·Ö±šŃ”ŌńĮĖČēĻĀµē¼«²ÄĮĻŗĶµē½āÖŹČÜŅŗ£ŗ
ijæĪĶā»ī¶ÆŠ”×é×¼±øÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆ½ųŠŠŹµŃ飮ĻÖÓŠ¼×”¢ŅŅ”¢±ūČżĪ»Ķ¬Ń§·Ö±šŃ”ŌńĮĖČēĻĀµē¼«²ÄĮĻŗĶµē½āÖŹČÜŅŗ£ŗ ÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆ½ųŠŠµē½ā£®AÖŠŹ¢ÓŠAgNO3ČÜŅŗ£¬BÖŠŹ¢ÓŠ±„ŗĶNa2SO4ČÜŅŗĶصēŅ»»į¶ł£¬·¢ĻÖŹŖČóµÄµķ·ŪKIŹŌÖ½µÄC¶Ė±äĪŖĄ¶É«£®Ōņ£ŗ
ÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆ½ųŠŠµē½ā£®AÖŠŹ¢ÓŠAgNO3ČÜŅŗ£¬BÖŠŹ¢ÓŠ±„ŗĶNa2SO4ČÜŅŗĶصēŅ»»į¶ł£¬·¢ĻÖŹŖČóµÄµķ·ŪKIŹŌÖ½µÄC¶Ė±äĪŖĄ¶É«£®Ōņ£ŗ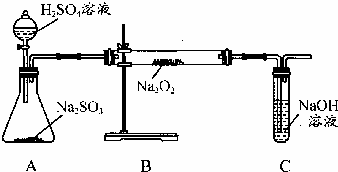 ij»ÆѧѧĻ°Š”×éµÄĶ¬Ń§ĪŖĢ½¾æ¹żŃõ»ÆÄĘÓė¶žŃõ»ÆĮņµÄ·“Ó¦£¬ÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆ½ųŠŠŹµŃ飮ĶØČė×ćĮæµÄĘųĢåŗ󽫓ų»šŠĒµÄľĢõ²åČėŹŌ¹ÜC֊ľĢõø“Č¼£®Ēė»Ų“šĻĀĮŠĪŹĢā
ij»ÆѧѧĻ°Š”×éµÄĶ¬Ń§ĪŖĢ½¾æ¹żŃõ»ÆÄĘÓė¶žŃõ»ÆĮņµÄ·“Ó¦£¬ÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆ½ųŠŠŹµŃ飮ĶØČė×ćĮæµÄĘųĢåŗ󽫓ų»šŠĒµÄľĢõ²åČėŹŌ¹ÜC֊ľĢõø“Č¼£®Ēė»Ų“šĻĀĮŠĪŹĢā