
 ����һ�ֿ����ھ�ˮ����ʳƷ���Σ���A��B��C��D��E���ֶ�����Ԫ����ɣ�������ˮ��ɵ�����������ӣ�����һ������A��B�γɵ�10���������ӣ�AԪ��ԭ�Ӻ�����������E����l��D��Eͬ���壮ijͬѧΪ̽������ɶ���������ʵ�飺
����һ�ֿ����ھ�ˮ����ʳƷ���Σ���A��B��C��D��E���ֶ�����Ԫ����ɣ�������ˮ��ɵ�����������ӣ�����һ������A��B�γɵ�10���������ӣ�AԪ��ԭ�Ӻ�����������E����l��D��Eͬ���壮ijͬѧΪ̽������ɶ���������ʵ�飺���� ����A��B��C��D��E���ֶ�����Ԫ����ɵ�һ���Σ���ȡ��������Һ���Թ��У���ε���Ba��OH��2��Һ�����ɳ�����ʼ���������С�����������ղ���ȫ��ʧ�������Һ�϶�����SO42-��Al3+����ȡ20mL����Һ���Թ��У��������NaOH��Һ����Ȳ��ռ����������壬�����Һ�к���NH4+�������ڵ��봦���������ӣ�A��B�γɵ�10����������ΪNH4+��D��Eͬ���壬����Ӧ�γ�SO42-����AԪ��ԭ�Ӻ�����������E����l����AΪNԪ�ء�EΪOԪ�ء�DΪSԪ�ء�BΪHԪ�ء�CΪAl������Һ����SO42-��Al3+��NH4+�������Ӻ����������ʵ���֮��1��1�����ԼĻ�ѧʽΪNH4Al��SO4��2•nH2O�����ⶨ�����Ħ������Ϊ453g��mol-1�����Լ��к��нᾧˮΪ$\frac{453-237}{18}$=12�����ԼĻ�ѧʽΪ��NH4Al��SO4��2•12H2O��oa�η�����Ӧ��2NH4Al��SO4��2+3Ba��OH��2=3BaSO4��+2Al��OH��3��+��NH4��2SO4��ab�η�����Ӧ����NH4��2SO4+Ba��OH��2=BaSO4��+2NH3��H2O��bc�η�����Ӧ��OH-+Al��OH��3=AlO2-+2H2O���ݴ˽��
��� �⣺����A��B��C��D��E���ֶ�����Ԫ����ɵ�һ���Σ���ȡ��������Һ���Թ��У���ε���Ba��OH��2��Һ�����ɳ�����ʼ���������С�����������ղ���ȫ��ʧ�������Һ�϶�����SO42-��Al3+����ȡ20mL����Һ���Թ��У��������NaOH��Һ����Ȳ��ռ����������壬�����Һ�к���NH4+�������ڵ��봦���������ӣ�A��B�γɵ�10����������ΪNH4+��D��Eͬ���壬����Ӧ�γ�SO42-����AԪ��ԭ�Ӻ�����������E����l����AΪNԪ�ء�EΪOԪ�ء�DΪSԪ�ء�BΪHԪ�ء�CΪAl������Һ����SO42-��Al3+��NH4+�������Ӻ����������ʵ���֮��1��1�����ԼĻ�ѧʽΪNH4Al��SO4��2•nH2O�����ⶨ�����Ħ������Ϊ453g��mol-1�����Լ��к��нᾧˮΪ$\frac{453-237}{18}$=12�����ԼĻ�ѧʽΪ��NH4Al��SO4��2•12H2O��oa�η�����Ӧ��2NH4Al��SO4��2+3Ba��OH��2=3BaSO4��+2Al��OH��3��+��NH4��2SO4��ab�η�����Ӧ����NH4��2SO4+Ba��OH��2=BaSO4��+2NH3��H2O��bc�η�����Ӧ��OH-+Al��OH��3=AlO2-+2H2O��
��1��DΪSԪ�أ��������ڱ��е�������VIA�壬
�ʴ�Ϊ����������VIA�壻
��2��������������֪���Ļ�ѧʽΪ��NH4Al��SO4��2•12H2O��
�ʴ�Ϊ��NH4Al��SO4��2•12H2O��
��3������NH4Al��SO4��2•12H2OΪ2mol��oa�η�����Ӧ��2NH4Al��SO4��2+3Ba��OH��2=3BaSO4��+2Al��OH��3��+��NH4��2SO4������3molBa��OH��2������1mol��NH4��2SO4������2molAl��OH��3��ab�η�����Ӧ����NH4��2SO4+Ba��OH��2=BaSO4��+2NH3��H2O��1mol��NH4��2SO4����1molBa��OH��2��bc�η�����Ӧ��OH-+Al��OH��3=AlO2-+2H2O��2molAl��OH��3����1molBa��OH��2����ͼ����V��Oa����V��ab����V��bc��=3mol��1mol��1mol=3��1��1��
�ʴ�Ϊ��3��1��1��
��4��ab�η�����Ӧ����NH4��2SO4+Ba��OH��2=BaSO4��+2NH3��H2O�����ӷ���ʽΪ��2NH4++SO42-+Ba2++2OH-=BaSO4��+2NH3��H2O��
�ʴ�Ϊ��2NH4++SO42-+Ba2++2OH-=BaSO4��+2NH3��H2O��
��5��ʵ�����ȡ20mL����Һ���Թ��У��������NaOH��Һ����Ȳ��ռ������İ���Ϊ$\frac{0.224L}{22.4L/mol}$=0.01mol����NH4Al��SO4��2Ϊ0.01mol������ҺŨ��Ϊ$\frac{0.01mol}{0.02L}$=0.5mol/L��
�ʴ�Ϊ��0.5mol/L��
���� ���⿼��������ƶϣ��ؼ��Ǹ���ʵ�������ƶϺ��е�������ȷ���η����ķ�Ӧ���Ƕ�ѧ���ۺ������Ŀ��飬�ѶȽϴ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
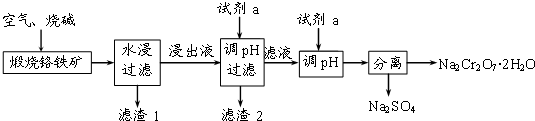
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��NH3���ȶ��Ա�PH3ǿ����д��ǿ������������
��NH3���ȶ��Ա�PH3ǿ����д��ǿ������������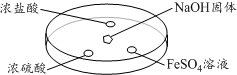
 NH3•H2O
NH3•H2O NH4++OH-������NaOH��OH-Ũ������ƽ�������ƶ����������ڰ����ѳ�����
NH4++OH-������NaOH��OH-Ũ������ƽ�������ƶ����������ڰ����ѳ������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ˮ�еμ�ŨH2SO4��KW���� | |
| B�� | ��NaAlO2��Һ�еμ�NaHCO3��Һ���г������������� | |
| C�� | �к͵���������ʵ���Ũ�ȵ�����ʹ��ᣬ�����ĵ��������Ƶ����ʵ�����ͬ | |
| D�� | NaCl��Һ��CH3COONH4��Һ�������ԣ�����Һ��ˮ�ĵ���̶���ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | SO3 | B�� | BF3 | C�� | CO32- | D�� | PH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ��������Ԫ���뱻��ԭ��Ԫ�ص�������Ϊ1��1 | |
| B�� | NaBH4�������������ǻ�ԭ�� | |
| C�� | NaBH4�ǻ�ԭ����ˮ�������� | |
| D�� | B��������H����ԭ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | �٢ڢܢ� | B�� | �ۢݢޢ� | C�� | �ڢܢݢ� | D�� | �٢ۢܢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com