
| ŹµŃé“ĪŹż | µŚŅ»“Ī | µŚ¶ž“Ī | µŚČż“Ī |
| ĻūŗÄøßĆĢĖį¼ŲČÜŅŗĢå»ż/mL | 10.32 | 10.02 | 9.98 |
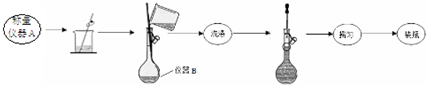
·ÖĪö £Ø1£©ÅäÖĘĦ¶ūŃĪČÜŅŗŹ±£¬³ĘČ”3.9200gĦ¶ūŃĪѳʷŅŖÓƵē×ÓĢģĘ½³ĘČ”£¬øł¾Ż×°ÖĆĶ¼æÉÖŖ£¬ÅäÖĘČÜŅŗ¶ØČŻŹ±ŅŖÓĆČŻĮæĘ棻
£Ø2£©½«ČŻĮæĘæÖŠŅŗĢåŅ”ŌČŅŖ°ŃČŻĮæĘæĘæČūČū½ō£¬ÓĆŹ³Öø¶„×”ĘæČū£¬ÓĆĮķŅ»Ö»ŹÖµÄŹÖÖøĶŠ×”Ęæµ×£¬°ŃČŻĮæĘæÉĻĻĀµßµ¹Ņ”¶Æ¶ą“Ī£¬Ź¹ČÜŅŗ»ģŗĻ¾łŌČ£¬¾Ż“Ė“šĢā£»
£Ø3£©øł¾ŻŹµŃ鶞¢ŪÖŠ²Ł×÷Į÷³ĢæÉÖŖ£¬°×É«Šü×ĒŅŗĪŖĮņĖį±µ£¬ŅŖ¾«Č·³ĘĮæĮņĖį±µµÄÖŹĮæŅŖ¾¹ż¹żĀĖ”¢Ļ“µÓ”¢øÉŌļŌŁ³ĘĮ棻
£Ø4£©ŹµŃ鶞¢ŪÖŠµĪ¶ØŹ±·¢Éś·“Ó¦ĪŖøßĆĢĖįøłĄė×ÓŃõ»ÆŃĒĢśĄė×Ó£¬µĪ¶ØÖÕµćŹ±ČÜŅŗ»į³öĻÖĒ³×ĻŗģÉ«£¬ĪŖøßĆĢĖį¼ŲČÜŅŗµÄŃÕÉ«£»
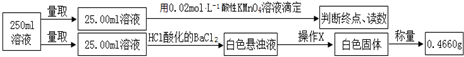
£Ø5£©øł¾ŻĢāÖŠŹµŃé²½Öč¼°Źż¾ŻæÉÖŖ£¬µĪ¶ØŃĒĢśĄė×ÓÓĆČ„µÄøßĆĢĖį¼ŲČÜŅŗµÄĢå»żµÄŹż¾ŻÖŠ£¬µŚŅ»“ĪĪó²ī½Ļ“ó£¬ĪŖÅ¼Č»Īó²īӦȄµō£¬ĖłŅŌøßĆĢĖį¼ŲČÜŅŗµÄĢå»żĪŖ$\frac{10.02+9.98}{2}$mL=10.00mL£¬øł¾ŻøßĆĢĖį¼ŲµÄĪļÖŹµÄĮææÉŅŌČ·¶ØŃĒĢśĄė×ÓµÄĪļÖŹµÄĮ棬øł¾Ż°×É«¹ĢĢå0.4660gæÉŅŌČ·¶ØĮņĖįøłĄė×ÓµÄĪļÖŹµÄĮ棬ĄūÓƵēŗÉŹŲŗćæÉÖŖļ§øłĄė×ÓµÄĪļÖŹµÄĮ棬ÓÉѳʷµÄÖŹĮæ¼°ĮņĖįøłĄė×Ó”¢ŃĒĢśĄė×Ó¼°ļ§øłĄė×ÓµÄÖŹĮæČ·¶Ø½į¾§Ė®µÄÖŹĮ棬¾Ż“ĖæÉČ·¶ØĦ¶ūŃĪµÄ×é³É£®
½ā“š ½ā£ŗ£Ø1£©ÅäÖĘĦ¶ūŃĪČÜŅŗŹ±£¬³ĘČ”3.9200gĦ¶ūŃĪѳʷŅŖÓƵē×ÓĢģĘ½³ĘČ”£¬øł¾Ż×°ÖĆĶ¼æÉÖŖ£¬ÅäÖĘ250mLČÜŅŗ¶ØČŻŹ±ŅŖÓĆ250mLČŻĮæĘ棬
¹Ź“š°øĪŖ£ŗµē×ÓĢģĘ½£»250mLČŻĮæĘ棻
£Ø2£©½«ČŻĮæĘæÖŠŅŗĢåŅ”ŌČŅŖ°ŃČŻĮæĘæĘæČūČū½ō£¬ÓĆŹ³Öø¶„×”ĘæČū£¬ÓĆĮķŅ»Ö»ŹÖµÄŹÖÖøĶŠ×”Ęæµ×£¬°ŃČŻĮæĘæÉĻĻĀµßµ¹Ņ”¶Æ¶ą“Ī£¬Ź¹ČÜŅŗ»ģŗĻ¾łŌČ£¬
¹Ź“š°øĪŖ£ŗ°ŃČŻĮæĘæĘæČūČū½ō£¬ÓĆŹ³Öø¶„×”ĘæČū£¬ÓĆĮķŅ»Ö»ŹÖµÄŹÖÖøĶŠ×”Ęæµ×£¬°ŃČŻĮæĘæÉĻĻĀµßµ¹Ņ”¶Æ¶ą“Ī£¬Ź¹ČÜŅŗ»ģŗĻ¾łŌČ£»
£Ø3£©øł¾ŻŹµŃ鶞¢ŪÖŠ²Ł×÷Į÷³ĢæÉÖŖ£¬°×É«Šü×ĒŅŗĪŖĮņĖį±µ£¬ŅŖ¾«Č·³ĘĮæĮņĖį±µµÄÖŹĮæŅŖ¾¹ż¹żĀĖ”¢Ļ“µÓ”¢øÉŌļŌŁ³ĘĮ棬
¹Ź“š°øĪŖ£ŗ¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ»ņŗęøÉ£ØĄäČ“£©£»
£Ø4£©ŹµŃ鶞¢ŪÖŠµĪ¶ØŹ±·¢Éś·“Ó¦ĪŖøßĆĢĖįøłĄė×ÓŃõ»ÆŃĒĢśĄė×Ó£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖMnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O£¬µĪ¶ØÖÕµćµÄĻÖĻóŹĒ×īŗóŅ»µĪČÜŅŗµĪČė£¬ČÜŅŗ³öĻÖĒ³×ĻŗģÉ«£¬30Ćė²»ĶŹÉ«£¬
¹Ź“š°øĪŖ£ŗMnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O£»×īŗóŅ»µĪČÜŅŗµĪČė£¬ČÜŅŗ³öĻÖĒ³×ĻŗģÉ«£¬30Ćė²»ĶŹÉ«£»
£Ø5£©øł¾ŻĢāÖŠŹµŃé²½Öč¼°Źż¾ŻæÉÖŖ£¬µĪ¶ØŃĒĢśĄė×ÓÓĆČ„µÄøßĆĢĖį¼ŲČÜŅŗµÄĢå»żµÄŹż¾ŻÖŠ£¬µŚŅ»“ĪĪó²ī½Ļ“ó£¬ĪŖÅ¼Č»Īó²īӦȄµō£¬ĖłŅŌøßĆĢĖį¼ŲČÜŅŗµÄĢå»żĪŖ$\frac{10.02+9.98}{2}$mL=10.00mL£¬øßĆĢĖį¼ŲµÄĪļÖŹµÄĮæĪŖ0.02mol/L”Į0.01L=0.0002mol£¬øł¾Ż·“Ó¦MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O£¬æÉÖŖ3.9200gĦ¶ūŃĪѳʷ֊ŃĒĢśĄė×ÓµÄĪļÖŹµÄĮæĪŖ0.0002mol”Į$\frac{250}{25}$”Į5=0.01mol£¬ĘäÖŹĮæĪŖ0.5600g£¬°×É«¹ĢĢåĮņĖį±µĪŖ0.4660g£¬ĖłŅŌ3.9200gĦ¶ūŃĪѳʷ֊ĮņĖįøłĄė×ÓµÄĪļÖŹµÄĮæĪŖ$\frac{0.4660}{233}$”Į$\frac{250}{25}$=0.02mol£¬ĘäÖŹĮæĪŖ1.9200g£¬øł¾ŻµēŗÉŹŲŗćæÉÖŖ3.9200gĦ¶ūŃĪѳʷ֊ļ§øłĄė×ÓµÄĪļÖŹµÄĮæĪŖ0.02mol”Į2-0.01mol”Į2=0.02mol£¬ĘäÖŹĮæĪŖ0.3600g£¬ĖłŅŌѳʷ֊½į¾§Ė®µÄĪļÖŹµÄĮæĪŖ$\frac{3.9200g-1.9200g-0.5600g-0.3600g}{18g/mol}$=0.06mol£¬ĖłŅŌĦ¶ūŃĪµÄ×é³ÉĪŖ£ØNH4£©2SO4•FeSO4•6H2O»ņ£ØNH4£©2Fe£ØSO4£©2•6H2O£¬
¹Ź“š°øĪŖ£ŗÄÜ£»£ØNH4£©2SO4•FeSO4•6H2O»ņ£ØNH4£©2Fe£ØSO4£©2•6H2O£®
µćĘĄ ±¾Ģāæ¼²é»Æѧ¹¤ŅÕĮ÷³Ģ”¢ĪļÖŹµÄ·ÖĄėĢį“攢Ńõ»Æ»¹ŌµĪ¶ØÓ¦ÓĆ”¢ČÜŅŗÅäÖĘµČ£¬Ē峞ŹµŃéŌĄķŹĒ½āĢāµÄ¹Ų¼ü£¬ŠčŅŖѧɜ¾ß±øŌśŹµµÄ»ł“”ÖŖŹ¶Óė×ŪŗĻŌĖÓĆÖŖŹ¶·ÖĪöĪŹĢā”¢½ā¾öĪŹĢāµÄÄÜĮ¦£¬Č·¶Øѳʷ×é³ÉŹ±×¢ŅāŹŲŗćĖ¼ĻėµÄŌĖÓĆ£¬ÄѶČÖŠµČ£®


 ĘŚÄ©³å“Ģ100·Ö““ŠĀ½š¾ķĶźČ«ŹŌ¾ķĻµĮŠ“š°ø
ĘŚÄ©³å“Ģ100·Ö““ŠĀ½š¾ķĶźČ«ŹŌ¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
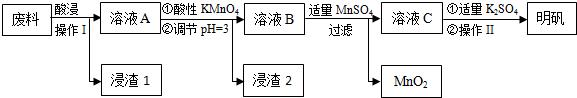
| Al£ØOH£©3 | Fe£ØOH£©2 | Fe£ØOH£©3 | |
| æŖŹ¼³ĮµķŹ± | 3.4 | 6.3 | 1.5 |
| ĶźČ«³ĮµķŹ± | 4.7 | 8.3 | 2.8 |
 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓĆĀČ»ÆøĘČÜŅŗ¼ų±šNa2CO3ŗĶNaHCO3Į½ÖÖČÜŅŗ | |
| B£® | ÓĆĒāŃõ»ÆÄĘČÜŅŗ¼ų±šMgCl2ČÜŅŗ”¢AlCl3ČÜŅŗ | |
| C£® | ĄūÓƶ”“ļ¶ūŠ§Ó¦¼ų±šFe£ØOH£©3½ŗĢåÓėFeCl3ČÜŅŗ | |
| D£® | ÓĆŃęÉ«·“Ó¦¼ų±šNaCl”¢KClŗĶNa2SO4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 0.46g | B£® | 0.69g | C£® | 0.92g | D£® | 0.23g |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | H2O2”śH2O | B£® | NH4+”śNH3 | C£® | Fe3+”śFe2+ | D£® | CO”śCO2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÄÜŹ¹¼×»ł³Č±äŗģµÄČÜŅŗÖŠ£ŗFe2+”¢Al3+”¢NO3-”¢Cl-”¢S2- | |
| B£® | ŌŚpH=11µÄČÜŅŗÖŠ£ŗNa+”¢AlO2-”¢NO3-”¢S2-”¢SO32- | |
| C£® | ŹŅĪĀĻĀ£¬ÓÉĖ®µēĄėµÄc£ØH+£©=10-10mol/LµÄČÜŅŗÖŠ£ŗCl-”¢HCO3-”¢NO3-”¢NH4+”¢F- | |
| D£® | 0.1 mol•L-1 FeCl3ČÜŅŗÖŠ£ŗK+”¢Na+”¢AlO2-”¢SCN- |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com