



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğÕć½Ź”Äž²ØŹŠŹ®Š£øßČż3ŌĀĮŖæ¼Ąķ×Ū»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
ÓŠĪåÖÖĦ¶ūÖŹĮæ¾łĪŖ44g/molµÄ»ÆŗĻĪļ¼×”¢ŅŅ”¢±ū”¢¶””¢Īģ£¬¾łÓɶĢÖÜĘŚŌŖĖŲ×é³É”£¶Ō¼×µÄ²¶»ńŌŚ½µµĶĪĀŹŅĘųĢåÅÅ·ÅÖŠ¾ßÓŠÖŲŅŖµÄ×÷ÓĆ”£ŅŅĪŖĢž£¬µČĪļÖŹµÄĮæµÄŅŅÓė¶”³ä·ÖČ¼ÉÕ²śĪļĪŖ¼×ÓėĖ®£¬ĒŅÉś³ÉĖ®µÄÖŹĮæĒ°ÕߏĒŗóÕßµÄ2±¶£¬±ūŌŚŅ»¶ØĢõ¼žĻĀ·Ö½ā»ńµĆĮ½ÖÖµ„ÖŹ£¬Į½µ„ÖŹ·ÅµēŹ±·“Ӧɜ³ÉA£¬AÓöæÕĘų±ä³Éŗģ×ŲÉ«ĘųĢåB”£ĪģŹĒŅ»ÖÖ¼«²»ĪČ¶ØµÄĪļÖŹ£¬ŌŚ1180”ęŅŌĻĀ²»“ęŌŚ”£æĘѧ¼ŅÓĆÖŹĘ×ŅĒŌŚ¹¤ŅµÖĘ¹čµÄ·“Ó¦²śĪļÖŠÖ¤ŹµĮĖĘä“ęŌŚ”£
£Ø1£©¶”µÄ·Ö×ÓŹ½????????????? £¬¼×µÄµē×ÓŹ½???????????????? ”£
£Ø2£©ČōÉś³ÉĪģµÄ·“Ó¦ÖŠŃõ»Æ²śĪļÓė»¹Ō²śĪļĪŖĶ¬Ņ»ĪļÖŹ£¬Š“³öÉś³ÉĪģµÄ»Æѧ·½³ĢŹ½???????? ”£
£Ø3£©°ŃĢśŗĶĶ»ģŗĶĪļ·ÅČėŅ»¶ØĮæBĶØČėĖ®ŗóŠĪ³ÉµÄĻ”ČÜŅŗÖŠ£¬·“Ó¦ŗó¹żĀĖ£¬ĀĖ³öµÄ¹ĢĢåĪļÖŹĶ¶ČėŃĪĖįÖŠĪŽĘųĢå·Å³ö£¬ŌņĀĖŅŗÖŠŅ»¶Øŗ¬ÓŠµÄČÜÖŹŹĒ????????????????????????????? ”£
£Ø4£©½«0.2molŅŅĶźČ«Č¼ÉÕŗóÉś³ÉµÄĘųĢåČ«²æ»ŗĀżĶØČė300mLijÅØ¶ČµÄNaOHČÜŅŗÖŠ£¬ĘųĢåĶźČ«±»ĪüŹÕ£¬ČÜŅŗÖŠNaOHĪŽŹ£Óą£¬ŌņNaOHČÜŅŗµÄÅضČĪŖ?????? (ČōÓŠ¶ØÖµŌņŠ“¾ßĢåŹżÖµ£¬ĪŽ¶ØÖµŌņŠ“·¶Ī§)”£
£Ø5£©½«µČĪļÖŹµÄĮæA”¢BµÄ»ģŗĻĪļČÜÓŚNaOHČÜŅŗÖŠµĆµ½Ö»ŗ¬ÓŠŅ»ÖÖČÜÖŹµÄČÜŅŗ£¬“ĖČÜÖŹµÄ»ÆѧŹ½ĪŖ????????????? £¬Éč¼Ę¼ņµ„ŹµŃé·½°øÖ¤Ć÷ČÜŅŗÖŠŗ¬ÓŠ“ĖČÜÖŹ?????????????????????? ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
 ijČÜŅŗÖŠæÉÄÜŗ¬ÓŠH+”¢Na+”¢
ijČÜŅŗÖŠæÉÄÜŗ¬ÓŠH+”¢Na+”¢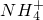 ”¢Mg2+”¢Fe3+”¢Al3+”¢
”¢Mg2+”¢Fe3+”¢Al3+”¢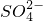 µČĄė×Ó£¬µ±ĻņøĆČÜŅŗÖŠ¼ÓČėijÅØ¶ČµÄNaOHČÜŅŗŹ±£¬·¢ĻÖÉś³É³ĮµķµÄĪļÖŹµÄĮæĖęNaOHČÜŅŗµÄĢå»ż±ä»ÆČēĶ¼ĖłŹ¾£¬ÓÉ“ĖæÉÖŖ£¬øĆČÜŅŗÖŠæĻ¶Øŗ¬ÓŠµÄŃōĄė×ÓŹĒH+”¢
µČĄė×Ó£¬µ±ĻņøĆČÜŅŗÖŠ¼ÓČėijÅØ¶ČµÄNaOHČÜŅŗŹ±£¬·¢ĻÖÉś³É³ĮµķµÄĪļÖŹµÄĮæĖęNaOHČÜŅŗµÄĢå»ż±ä»ÆČēĶ¼ĖłŹ¾£¬ÓÉ“ĖæÉÖŖ£¬øĆČÜŅŗÖŠæĻ¶Øŗ¬ÓŠµÄŃōĄė×ÓŹĒH+”¢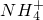 ”¢Mg2+”¢Al3+
”¢Mg2+”¢Al3+²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğ½ĖÕŹ”ĪŽĪżŹŠ½ŅõŹŠ×£ĢĮ֊ѧø߶ž£ØÉĻ£©ĘŚÖŠ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
 ijČÜŅŗÖŠæÉÄÜŗ¬ÓŠH+”¢Na+”¢
ijČÜŅŗÖŠæÉÄÜŗ¬ÓŠH+”¢Na+”¢ ”¢Mg2+”¢Fe3+”¢Al3+”¢
”¢Mg2+”¢Fe3+”¢Al3+”¢ µČĄė×Ó£¬µ±ĻņøĆČÜŅŗÖŠ¼ÓČėijÅØ¶ČµÄNaOHČÜŅŗŹ±£¬·¢ĻÖÉś³É³ĮµķµÄĪļÖŹµÄĮæĖęNaOHČÜŅŗµÄĢå»ż±ä»ÆČēĶ¼ĖłŹ¾£¬ÓÉ“ĖæÉÖŖ£¬øĆČÜŅŗÖŠæĻ¶Øŗ¬ÓŠµÄŃōĄė×ÓŹĒH+”¢
µČĄė×Ó£¬µ±ĻņøĆČÜŅŗÖŠ¼ÓČėijÅØ¶ČµÄNaOHČÜŅŗŹ±£¬·¢ĻÖÉś³É³ĮµķµÄĪļÖŹµÄĮæĖęNaOHČÜŅŗµÄĢå»ż±ä»ÆČēĶ¼ĖłŹ¾£¬ÓÉ“ĖæÉÖŖ£¬øĆČÜŅŗÖŠæĻ¶Øŗ¬ÓŠµÄŃōĄė×ÓŹĒH+”¢ ”¢Mg2+”¢Al3+
”¢Mg2+”¢Al3+²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗĘŚÄ©Ģā ĢāŠĶ£ŗµ„Ń”Ģā
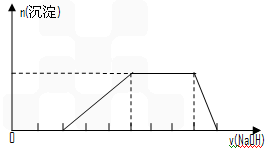
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com