
��̼��������ֱ�Ӻϳ��Ҵ�ȼ���ѽ�����ģ������
��Ŀǰ��ҵ�Ͽ�����CO2������CH3CH2OH����ӦΪ��2CO2(g)+6H2(g)![]() CH3CH2OH(g)+H2O(g)����һ��ѹǿ�£��÷�Ӧ��һЩʵ���������±���
CH3CH2OH(g)+H2O(g)����һ��ѹǿ�£��÷�Ӧ��һЩʵ���������±���
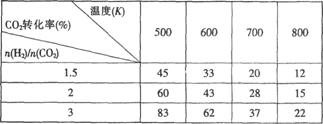
���ݱ������ݷ�����
��1���÷�Ӧ������Ӧ��_________��ѡ����ȡ����ȡ�����Ӧ��
��2�������̼��n(H2)/n(CO2)���������Ҵ� _______��ѡ���������������������Ӱ�족����
��3�������Ϊ1L�ĺ����ܱ������У�����1molCO2��3molH2�����д�ʩ����ʹc(CH3CH2OH)�������_______������ĸ����ͬ����
A�������¶� B������He(g)��ʹ��ϵѹǿ����
C����H2O(g)����ϵ�з������ D���ٳ���1molCO2��3molH2
��ҵ�ϻ���ȡ��CO�ͣ���Ϊԭ�Ϻϳ��Ҵ����仯ѧ��Ӧ����ʽΪ��
2CO(g)+4H2(g)![]() CH3CH2OH(g)+H2O(g)
CH3CH2OH(g)+H2O(g)
��4����д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK= ___________��
��5��һ���¶��£���һ���ݻ��ɱ���ܱ������У�����������Ӧ���������жϸ÷�Ӧ�ﵽ��ѧƽ��״̬����___________��
A��c(CO)=c(H2) B��v��(CO)=v��(H2O)
C�������е�ѹǿ���� D������2molCO��ͬʱ����1molCH3CH2OH
��6������ͬ�����£���CO��ȡCH3CH2OH��ƽ�ⳣ��ԶԶ������CO2��ȡCH3CH2OH��ƽ�ⳣ��������CO��ȡCH3CH2OH���ŵ���______________________����CO2��ȡCH3CH2OH���ŵ���____________����д��һ�㼴�ɣ�
 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| c(CH3CH2OH)?c(H2O) |
| c2(CO)?c4(H2) |
| c(CH3CH2OH)?c(H2O) |
| c2(CO)?c4(H2) |
| �¶ȣ�K�� CO2ת���ʣ�%�� n��H2��/n��CO2�� |
500 | 600 | 700 | 800 |
| 1.5 | 45 | 33 | 20 | 12 |
| 2 | 60 | 43 | 28 | 15 |
| 3 | 83 | 62 | 37 | 22 |
| n(H2) |
| n(CO2) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��̼��������ֱ�Ӻϳɼ״����Ҵ�ȼ���ѽ��빤ҵ�������磺
��̼��������ֱ�Ӻϳɼ״����Ҵ�ȼ���ѽ��빤ҵ�������磺| n(CO) |
| n(H2) |
| ʵ����� | T���棩 |
|
p��Mpa�� | CO��ת���ʣ� | ||
| 1 | 150 |
|
0.1 | a | ||
| 2 | x |
|
5 | b |
| n(CO) |
| n(H2) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��̼��������ֱ�Ӻϳ��Ҵ�ȼ���ѽ�����ģ��������ͼ���ɶ�����̼�ϳ��Ҵ��ļ������̣�
���ճ���ʢ�б���̼�����Һ���Ѻ��ж�����̼�Ŀ����������ճ��У����ճ��з�ӦҺ����ֽ�غ���ֽ����ͨ�����ˮ�������Ѷ�����̼����Һ����ȡ�������ںϳ����к���������ѧ��Ӧʹ֮��Ϊ������ȼ���Ҵ����ش��������⣺
��1��д�����ճ��з�Ӧ�����ӷ���ʽ ��
��2���ӷֽ����ѭ��ʹ�õ������� ��
��3����ҵ�ϻ���ȡ��CO�ͣ���Ϊԭ�Ϻϳ��Ҵ����仯ѧ��Ӧ����ʽΪ��
2CO(g)+4H2(g)CH3CH2OH(g)+H2O(g)
д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ K= ��
��4������ͬ�����£���CO��ȡCH3CH2OH��ƽ�ⳣ��ԶԶ������CO2��ȡCH3CH2OH ��ƽ�ⳣ��������CO��ȡCH3CH2OH���ŵ���ʹԭ���нϴ��ת���ʣ���CO2��ȡCH3CH2OH���ŵ��� ����д��һ�㼴�ɣ�
|
| 500 | 600 | 700 | 800 |
| 1.5 | 45 | 33 | 20 | 12 |
| 2.0 | 60 | 43 | 28 | 15 |
| 3.0 | 83 | 62 | 37 | 22 |
��5����һ��ѹǿ�£������CO2��ȡCH3CH2OH��ʵ���������±���
���ݱ������ݷ�����
���¶����ߣ��÷�Ӧ��ƽ�ⳣ��Kֵ ��ѡ���������С�����䡱����
�������̼n(H2)/n(CO2)��,�������Ҵ� ��ѡ���������������������Ӱ�족����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��㶫ʡ��ͷ�и�����һ��ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ������
��̼��������ֱ�Ӻϳ��Ҵ�ȼ���ѽ�����ģ��������ͼ���ɶ�����̼�ϳ��Ҵ��ļ������̣�
���ճ���ʢ�б���̼�����Һ���Ѻ��ж�����̼�Ŀ����������ճ��У����ճ��з�ӦҺ����ֽ�غ���ֽ����ͨ�����ˮ�������Ѷ�����̼����Һ����ȡ�������ںϳ����к���������ѧ��Ӧʹ֮��Ϊ������ȼ���Ҵ����ش��������⣺
��1��д�����ճ��з�Ӧ�����ӷ���ʽ ��
��2���ӷֽ����ѭ��ʹ�õ������� ��
��3����ҵ�ϻ���ȡ��CO�ͣ���Ϊԭ�Ϻϳ��Ҵ����仯ѧ��Ӧ����ʽΪ��
2CO(g)+4H2(g) CH3CH2OH(g)+H2O(g)
CH3CH2OH(g)+H2O(g)
д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ K= ��
��4������ͬ�����£���CO��ȡCH3CH2OH��ƽ�ⳣ��ԶԶ������CO2��ȡCH3CH2OH��ƽ�ⳣ��������CO��ȡCH3CH2OH���ŵ���ʹԭ���нϴ��ת���� ����CO2��ȡCH3CH2OH���ŵ��� ����д��һ�㼴�ɣ�
 | 500 | 600 | 700 | 800 |
| 1.5 | 45 | 33 | 20 | 12 |
| 2.0 | 60 | 43 | 28 | 15 |
| 3.0 | 83 | 62 | 37 | 22 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ�߶���ѧ�����п������⻯ѧ ���ͣ������
��̼��������ֱ�Ӻϳ��Ҵ�ȼ���ѽ�����ģ������
��Ŀǰ��ҵ�Ͽ�����CO2������CH3CH2OH����ӦΪ��2CO2(g)+6H2(g) CH3CH2OH(g)+H2O(g)����һ��ѹǿ�£��÷�Ӧ��һЩʵ���������±���
CH3CH2OH(g)+H2O(g)����һ��ѹǿ�£��÷�Ӧ��һЩʵ���������±���

���ݱ������ݷ�����
��1���÷�Ӧ������Ӧ��_________��ѡ����ȡ����ȡ�����Ӧ��
��2�������̼��n(H2)/n(CO2)���������Ҵ� _______��ѡ���������������������Ӱ�족����
��3�������Ϊ1L�ĺ����ܱ������У�����1molCO2��3molH2�����д�ʩ����ʹc(CH3CH2OH)�������_______������ĸ����ͬ����
A�������¶� B������He(g)��ʹ��ϵѹǿ����
C����H2O(g)����ϵ�з������ D���ٳ���1molCO2��3molH2
��ҵ�ϻ���ȡ��CO�ͣ���Ϊԭ�Ϻϳ��Ҵ����仯ѧ��Ӧ����ʽΪ��
2CO(g)+4H2(g) CH3CH2OH(g)+H2O(g)
CH3CH2OH(g)+H2O(g)
��4����д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK= ___________��
��5��һ���¶��£���һ���ݻ��ɱ���ܱ������У�����������Ӧ���������жϸ÷�Ӧ�ﵽ��ѧƽ��״̬����___________��
A��c(CO)=c(H2) B��v��(CO)=v��(H2O)
C�������е�ѹǿ���� D������2molCO��ͬʱ����1molCH3CH2OH
��6������ͬ�����£���CO��ȡCH3CH2OH��ƽ�ⳣ��ԶԶ������CO2��ȡCH3CH2OH��ƽ�ⳣ��������CO��ȡCH3CH2OH���ŵ���______________________����CO2��ȡCH3CH2OH���ŵ���____________����д��һ�㼴�ɣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com