
| µĪ¶Ø“ĪŹż | ŃĪĖįĢå»ż£ØmL£© | NaOHČÜŅŗĢå»ż¶ĮŹż£ØmL£© | |
| µĪ¶ØĒ° | µĪ¶Øŗó | ||
| 1 | 20.00 | 0.00 | 18.10 |
| 2 | 20.00 | 0.00 | 16.30 |
| 3 | 20.00 | 0.00 | 16.22 |


 ČŹ°®Ó¢ÓļĶ¬²½Į·Ļ°²įĻµĮŠ“š°ø
ČŹ°®Ó¢ÓļĶ¬²½Į·Ļ°²įĻµĮŠ“š°ø ѧĻ°Źµ¼łŌ°µŲĻµĮŠ“š°ø
ѧĻ°Źµ¼łŌ°µŲĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
| ŹµŃ鱹ŗÅ | IØCµÄ³õŹ¼ÅØ¶Č (mol”¤L-1) | OClØCµÄ³õŹ¼ÅØ¶Č (mol”¤L-1) | OHØCµÄ³õŹ¼ÅØ¶Č (mol”¤L-1) | ³õŹ¼ĖŁĀŹv (mol”¤L-1”¤ s-1) |
| 1 | 2 ”Į 10ØC3 | 1.5 ”Į 10ØC3 | 1.00 | 1.8 ”Į 10ØC4 |
| 2 | a | 1.5 ”Į 10ØC3 | 1.00 | 3.6 ”Į 10ØC4 |
| 3 | 2 ”Į 10ØC3 | 3 ”Į 10ØC3 | 2.00 | 1.8 ”Į 10ØC4 |
| 4 | 4 ”Į 10ØC3 | 3 ”Į 10ØC3 | 1.00 | 7.2 ”Į 10ØC4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
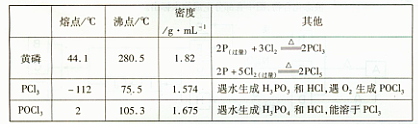
 5.00mL¼ÓČė¹żĮæµÄc1mol”¤L-lV1mLµāČÜŅŗ£¬³ä·Ö·“Ó¦ŗóŌŁÓĆc2mol”¤L-1Na2S2O3ČÜŅŗµĪ¶Ø¹żĮæµÄµā£¬ÖÕµćŹ±ĻūŗÄV2mLNa2S2O3ČÜŅŗ”£
5.00mL¼ÓČė¹żĮæµÄc1mol”¤L-lV1mLµāČÜŅŗ£¬³ä·Ö·“Ó¦ŗóŌŁÓĆc2mol”¤L-1Na2S2O3ČÜŅŗµĪ¶Ø¹żĮæµÄµā£¬ÖÕµćŹ±ĻūŗÄV2mLNa2S2O3ČÜŅŗ”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
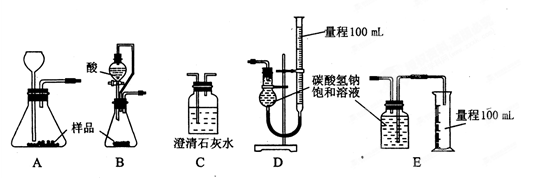
| A£®·ÖŅŗŹ±£¬·ÖŅŗĀ©¶·ĻĀ²ćŅŗĢå“ÓĻĀæŚ·Å³ö£¬ÉĻ²ćŅŗĢå“ÓÉĻæŚµ¹³ö |
| B£®ĢįČ”µāĖ®ÖŠµÄµāµ„ÖŹŹ±£¬Ó¦Ń”ŌńÓŠ»śŻĶČ”¼Į£¬ĒŅŻĶČ”¼ĮµÄĆܶȱŲŠė±ČĖ®“ó |
| C£®ÓĆPHŹŌÖ½²ā¶ØijĪŽÉ«ČÜŅŗµÄPHŹ±£¬Ó¦½«PHŹŌÖ½·ÅČėČÜŅŗÖŠ£¬¹Ū²ģĘäŃÕÉ«±ä»Æ£¬øś±ź×¼±ČÉ«æØ±Č½Ļ |
| D£®ÕōĮó²Ł×÷ÖŠ£¬ĪĀ¶Č¼ĘÓ¦øĆ·ÅŌŚÕōĮóÉÕĘæÄŚµÄŅŗĢåÖŠ£¬ŅŌ²āĮæŅŗĢåµÄĪĀ¶Č |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
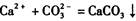 µÄ·“Ó¦Éč¼ĘĮĖČēĻĀ·½°ø:³ĘȔѳʷ”śÅä³ÉČÜŅŗ”ś¼ÓČė×ćĮæÅØCaCl2ČÜŅŗ”ś³ä·Ö·“Ó¦ŗó¹żĀĖ”śĻ“µÓ”śøÉŌļ”ś³ĘĮæ”ś¼ĘĖć“æ¶Č”£
µÄ·“Ó¦Éč¼ĘĮĖČēĻĀ·½°ø:³ĘȔѳʷ”śÅä³ÉČÜŅŗ”ś¼ÓČė×ćĮæÅØCaCl2ČÜŅŗ”ś³ä·Ö·“Ó¦ŗó¹żĀĖ”śĻ“µÓ”śøÉŌļ”ś³ĘĮæ”ś¼ĘĖć“æ¶Č”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ČŻĮæĘæÖŠŌÓŠÉŁĮæÕōĮóĖ® | B£®ČܽāĖłÓƵÄÉÕ±Ī“Ļ“µÓ |
| C£®¶ØČŻŹ±ŃöŹÓ¹Ū²ģŅŗĆę | D£®¶ØČŻŹ±ø©ŹÓ¹Ū²ģŅŗĆę |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ÓĆŹ³ŃĪĖ®³żĖ®¹ø |
| B£®ÓĆ¼ÓČȵķ½·Øøų¾ŪŅŅĻ©ĖÜĮĻ“ü·āæŚ |
| C£®ÓĆĒ¦±ŹŠ¾“śĢęŹÆÄ«ŹŌŃéµ¼µēŠŌ |
| D£®ÓĆ×ĘÉյķ½·ØĒų±šŃņĆ«ĻßŗĶĆŽĻß |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Ä³¹ĢĢå¼ÓČėĻ”ŃĪĖį,²śÉśĪŽÉ«ĒŅŹ¹³ĪĒåŹÆ»ŅĖ®±ä»ė×ĒµÄĘųĢå,Ö¤Ć÷øĆ¹ĢĢåŅ»¶Øŗ¬CO32- |
| B£®¼ģŃéÉÕ¼īČÜŅŗÖŠŹĒ·ńŗ¬Cl-£¬ĻČ¼ÓĻ”ŃĪĖį£¬ŌŁ¼ÓAgNO3ČÜŅŗ£¬²śÉś°×É«³Įµķ |
C£®Ä³ČÜŅŗÖŠµĪ¼ÓBaCl2ČÜŅŗ£¬ŌŁ¼ÓĻ”HNO3£¬²śÉś°×É«³Įµķ£¬ŌČÜŅŗÖŠŅ»¶Øŗ¬SO |
| D£®Ä³ČÜŅŗÖŠµĪČėKSCNČÜŅŗĪŽĆ÷ĻŌ±ä»Æ£¬ŌŁ¼ÓČėĀČĖ®³ŹŗģÉ«£¬ŌČÜŅŗÖŠŅ»¶Øŗ¬Fe2+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
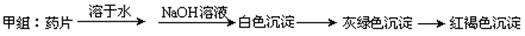 ¼××éĶ¬Ń§°“ÕÕÉč¼ĘµÄ·½°øĶź³ÉŹµŃ飬µ«ŅÅŗ¶µÄŹĒĖūĆĒƻӊµĆµ½Ō¤ĘŚµÄŹµŃé½į¹ū£¬
¼××éĶ¬Ń§°“ÕÕÉč¼ĘµÄ·½°øĶź³ÉŹµŃ飬µ«ŅÅŗ¶µÄŹĒĖūĆĒƻӊµĆµ½Ō¤ĘŚµÄŹµŃé½į¹ū£¬ 
 ½ųŠŠŹµŃéŃéÖ¤£ŗ________________________________
½ųŠŠŹµŃéŃéÖ¤£ŗ________________________________| ŠņŗÅ | V£ØKMnO4£©³õ | V£ØKMnO4£©ÖÕ | V£ØKMnO4£© |
| 1 | 2.24mL | 14.25mL | 12.01mL |
| 2 | 0.30mL | 12.72mL | 12.42mL |
| 3 | 0.50mL | 12.53 | 12.03mL |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com