
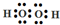 £»
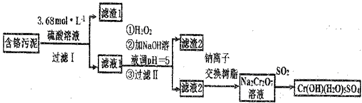
£»·ÖĪö ŗ¬øõĪŪÄą¼ÓČė3.68mol/LµÄĮņĖįČÜŅŗ£¬¹żĀĖµĆµ½ĮņĖį½žČ”ŅŗÖŠµÄ½šŹōĄė×ÓÖ÷ŅŖŹĒCr3+£¬Ęä“ĪŹĒFe3+”¢Al3+”¢Ca2+£¬¼ÓČė¹żŃõ»ÆĒā£¬H2O2µÄ×÷ÓĆŹĒ½«ĀĖŅŗ¢ńÖŠµÄCr3+×Ŗ»ÆĪŖCr2O72-£¬¼ÓČėNaOHČÜŅŗŹ¹ČÜŅŗ³ŹĖįŠŌ£¬PH=5“ĖŹ±ĢśĄė×Ó”¢ĀĮĄė×Ó£¬
£Ø1£©øł¾Żc=$\frac{1000¦Ńw}{M}$¼ĘĖćÅØĮņĖįµÄĪļÖŹµÄĮæÅØ¶Č£¬ŌŁøł¾ŻĻ”ŹĶ¶ØĀɼĘĖćŠčŅŖÅØĮņĖįµÄĢå»ż£»
£Ø2£©¹żŃõ»ÆĒāĪŖ¹²¼Ū»ÆŗĻĪļ£¬ŃõŌ×Ó¼äŠĪ³ÉŅ»øö¹²¼Ū¼ü£¬ĆæøöŃõŌ×ÓŗĶĮ½øöĒāŌ×Ó½įŗĻŠĪ³É¹²¼Ū¼ü£¬¾Ż“ĖŹéŠ“µē×ÓŹ½£»
£Ø3£©ĀĖŌü2ÖŠµÄ2ÖÖ»Æѧ³É·ÖĪŖĒāŃõ»ÆĀĮŗĶĒāŃõ»ÆĢś³Įµķ£¬·ÖĄė¶žÕߏĒĄūÓĆĒāŃõ»ÆĀĮĪŖĮ½ŠŌĒāŃõ»ÆĪļ£¬¼ÓČėĒāŃõ»ÆÄĘČÜŅŗČܽāĒāŃõ»ÆĀĮ£¬¹żĀĖµĆµ½ĒāŃõ»ÆĢś³Įµķ£¬ĀĖŅŗÖŠĶØČė¶žŃõ»ÆĢ¼Éś³ÉĒāŃõ»ÆĀĮ³Įµķ£»
£Ø4£©ĶعżÄĘĄė×Ó½»»»Ź÷Ö¬£¬³żČ„øĘĄė×Ó£»
£Ø5£©ĶØČėSO2¹ż³Ģ·¢Éś·“Ńõ»Æ»¹ŌӦɜ³ÉCr£ØOH£©£ØH2O£©5 SO4ŗĶNa2SO4£¬½įŗĻŌ×ÓŹŲŗćÅäĘ½ŹéŠ“»Æѧ·½³ĢŹ½£»
£Ø6£©Fe×öµē¼«£¬ĄūÓƵē½ā·Ø½«Na2Cr2O7×Ŗ»ÆĪŖCr£ØOH£©3³Įµķ£¬Ķ¬Ź±ÓŠFe£ØOH£©3³ĮµķÉś³É£¬Ńō¼«ĢśŹ§µē×ÓÉś³ÉŃĒĢśĄė×Ó£¬Ņõ¼«ĒāĄė×ӵƵ½µē×ÓÉś³ÉĒāĘų£¬ÖŲøõĖįÄĘŃõ»ÆŃĒĢśĄė×ÓÉś³ÉĢśĄė×ÓŌŚČÜŅŗÖŠÉś³ÉĒāŃõ»ÆĢś³Įµķ£¬ÖŲøõĖįøłĄė×Ó±»»¹ŌĪŖøõĄė×ÓÉś³ÉĒāŃõ»Æøõ³Įµķ£¬½įŗĻµēŗÉŹŲŗćŗĶŌ×ÓŹŲŗćÅäĘ½ŹéŠ“Ąė×Ó·½³ĢŹ½£¬½įŗĻĄė×Ó·½³ĢŹ½¼ĘĖćµē×Ó×ŖŅĘ£¬¼ĘĖ擦Ąķ1mol Na2Cr2O7ĄķĀŪÉĻŠčŅŖŹ±¼ä£¬µ±Na2Cr2O7ÅØ¶Č½Ļ“óŹ±Čż¼ŪĢśÄܹ»Č«²æ³Įµķ£¬¶ų²æ·ÖČż¼ŪøõŅŌijÖÖŠĪŹ½ČÜÓŚĖ®£¬¼īŠŌČÜŅŗÖŠĒāŃõ»ÆøõæÉÄÜ»įČÜÓŚ¼īÉś³ÉČÜÓŚĖ®µÄČÜŅŗ£®
½ā“š ½ā£ŗ£Ø1£©ÖŹĮæ·ÖŹżĪŖ98%£¬ĆܶČĪŖ1.84g/cm3µÄÅØĮņĖįµÄĪļÖŹµÄĮæÅضČ=$\frac{1000”Į1.84”Į98%}{98}$mol/L=18.4mol/L£¬
ĮīŠčŅŖÅØĮņĖįµÄĢå»żĪŖV£¬øł¾ŻĻ”ŹĶ¶ØĀÉ£¬Ļ”ŹĶĒ°ŗóČÜÖŹĮņĖįµÄĪļÖŹµÄĮæ²»±ä£¬Ōņ£ŗ
250mL”Į3.68mol/L=V”Į18.4mol/L
½āµĆV=50.0mL
¹Ź“š°øĪŖ£ŗ50.0£»
£Ø2£©¹żŃõ»ÆĒāĪŖ¹²¼Ū»ÆŗĻĪļ£¬ŃõŌ×Ó¼äŠĪ³ÉŅ»øö¹²¼Ū¼ü£¬ĆæøöŃõŌ×ÓŗĶĮ½øöĒāŌ×Ó½įŗĻŠĪ³É¹²¼Ū¼ü£¬H2O2µÄµē×ÓŹ½ĪŖ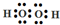 £¬
£¬
¹Ź“š°øĪŖ£ŗ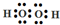 £»
£»
£Ø3£©ĀĖŌü2ÖŠµÄ2ÖÖ»Æѧ³É·ÖĪŖĒāŃõ»ÆĀĮŗĶĒāŃõ»ÆĢś³Įµķ£¬·ÖĄė¶žÕߏĒĄūÓĆĒāŃõ»ÆĀĮĪŖĮ½ŠŌĒāŃõ»ÆĪļ£¬¼ÓČėĒāŃõ»ÆÄĘČÜŅŗČܽāĒāŃõ»ÆĀĮ£¬¹żĀĖµĆµ½ĒāŃõ»ÆĢś³Įµķ£¬ĀĖŅŗÖŠĶØČė¶žŃõ»ÆĢ¼Éś³ÉĒāŃõ»ÆĀĮ³Įµķ£¬
¹Ź“š°øĪŖ£ŗCO2£»
£Ø4£©ĶعżÄĘĄė×Ó½»»»Ź÷Ö¬£¬³żČ„øĘĄė×Ó£¬øĘŌŖĖŲŌŚµŚ¢ņA£¬
¹Ź“š°øĪŖ£ŗ¢ņA£»
£Ø5£©ĶØČėSO2¹ż³Ģ·¢Éś·“Ńõ»Æ»¹ŌÓ¦£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗNa2Cr2O7+3SO2+11H2O=2Cr£ØOH£©£ØH2O£©5 SO4+Na2SO4£¬
¹Ź“š°øĪŖ£ŗNa2Cr2O7+3SO2+11H2O=2Cr£ØOH£©£ØH2O£©5 SO4+Na2SO4£»
£Ø6£©ÓĆFe×öµē¼«£¬ĄūÓƵē½ā·Ø½«Na2Cr2O7×Ŗ»ÆĪŖCr£ØOH£©3³Įµķ£¬Ķ¬Ź±ÓŠFe£ØOH£©3³ĮµķÉś³É£¬Ńō¼«ĢśŹ§µē×ÓÉś³ÉŃĒĢśĄė×Ó£¬Ņõ¼«ĒāĄė×ӵƵ½µē×ÓÉś³ÉĒāĘų£¬ÖŲøõĖįÄĘŃõ»ÆŃĒĢśĄė×ÓÉś³ÉĢśĄė×ÓŌŚČÜŅŗÖŠÉś³ÉĒāŃõ»ÆĢś³Įµķ£¬ÖŲøõĖįøłĄė×Ó±»»¹ŌĪŖøõĄė×ÓÉś³ÉĒāŃõ»Æøõ³Įµķ£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗ6Fe+19H2O+Cr2O72-=6H2+2Cr£ØOH£©3+6Fe£ØOH£©3+2OH-£¬µē½ā¹ż³ĢÖŠµē¼«ÉĻµē×Ó×ŖŅĘ×ÜŹż12e-£¬Čōµē½ā¹ż³ĢÖŠµēĮ÷Ēæ¶ČĪŖI£¬“¦Ąķ1mol Na2Cr2O7ĄķĀŪÉĻŠčŅŖŹ±¼äĪŖ$\frac{12q{N}_{A}}{I}$£¬µ±Na2Cr2O7ÅØ¶Č½Ļ“óŹ±Čż¼ŪĢśÄܹ»Č«²æ³Įµķ£¬¶ų²æ·ÖČż¼ŪøõŅŌijÖÖŠĪŹ½ČÜÓŚĖ®£¬¼īŠŌČÜŅŗÖŠĒāŃõ»Æøõ¾ßÓŠĮ½ŠŌæÉÄÜ»įČÜÓŚ¼īÉś³ÉČÜŅŗ£¬
¹Ź“š°øĪŖ£ŗ6Fe+19H2O+Cr2O72-=6H2+2Cr£ØOH£©3+6Fe£ØOH£©3+2OH-£»$\frac{12q{N}_{A}}{I}$£»Cr£ØOH£©3³ĮµķæÉÄÜÓėOH-·“Ó¦£Ø»ņCr£ØOH£©3¾ßÓŠĮ½ŠŌ£©£»
µćĘĄ ±¾Ģāæ¼²éĮĖĪļÖŹ·ÖĄėĢį“æµÄ·½·ØŗĶŹµŃ黳±¾²Ł×÷”¢µē½āŌĄķµÄ·ÖĪöÅŠ¶Ļ”¢×¢Ņā»Æѧ·½³ĢŹ½ŗĶĄė×Ó·½³ĢŹ½µÄŹéŠ“ŗĶ“ķĪó·ÖĪö£¬ÕĘĪÕ»ł“”ŹĒ½āĢā¹Ų¼ü£¬ĢāÄæÄѶČÖŠµČ£®


 ѧĮ·æģ³µµĄæģĄÖ¼ŁĘŚŗ®¼Ł×÷ŅµĻµĮŠ“š°ø
ѧĮ·æģ³µµĄæģĄÖ¼ŁĘŚŗ®¼Ł×÷ŅµĻµĮŠ“š°ø ŠĀĖ¼Ī¬ŗ®¼Ł×÷ŅµĻµĮŠ“š°ø
ŠĀĖ¼Ī¬ŗ®¼Ł×÷ŅµĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĮņĖįĒāÄĘÓėæĮŠŌ¼ŲČÜŅŗ·“Ó¦ | B£® | “×ĖįŗĶĒāŃõ»Æ¼ŲČÜŅŗ·“Ó¦ | ||
| C£® | Ļ”ĮņĖįŗĶ°±Ė®·“Ó¦ | D£® | Ģ¼ĖįĒāÄĘČÜŅŗÓėÉÕ¼īČÜŅŗ·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ź¹·ÓĢŖ±äŗģµÄČÜŅŗ£ŗK+”¢Na+”¢NO3-”¢Cl- | |
| B£® | ĪŽÉ«ĶøĆ÷µÄĖįŠŌČÜŅŗ£ŗMnO4-”¢K+”¢C1-”¢SO42- | |
| C£® | µĪ¼ÓKSCNĻŌŗģÉ«µÄČÜŅŗ£ŗNH4+”¢K+”¢Cl-”¢I- | |
| D£® | 0.1 mol•L-1NaHCO3ČÜŅŗ£ŗNa+”¢Ba2+”¢NO3-”¢OH- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | æÉÓĆÄ„æŚ²£Į§Ęæ±£“ęNaOHČÜŅŗ | |
| B£® | ĻņNa2SiO3ČÜŅŗÖŠĶØČė¹żĮæSO2£ŗSiO32-+SO2+H2OØTH2SiO3”ż+SO32- | |
| C£® | ¹č½ŗæÉ×÷“ü×°Ź³Ę·µÄøÉŌļ¼Į | |
| D£® | ²£Į§ČŻĘ÷æɳ¤ĘŚŹ¢·Åø÷ÖÖĖį |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | øĆŌ×ÓµÄĦ¶ūÖŹĮæŹĒaNA | |
| B£® | WgøĆŌ×ÓµÄĪļÖŹµÄĮæŅ»¶ØŹĒ$\frac{W}{aNA}$mol | |
| C£® | øĆŌ×ÓµÄĻą¶ŌŌ×ÓÖŹĮæŹĒ$\frac{12}{a}$ | |
| D£® | ÓÉŅŃÖŖŠÅĻ¢æÉµĆ£ŗNA=$\frac{12}{a}$ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Ń”Ļī | ĆčŹö | ½āŹĶ |
| A | ·²»šŅ©£¬ĮņĪŖ“æŃō£¬ĻõĪŖ“æŅõ£¬“ĖĒ¬Ą¤»Ć³öÉńĪļŅ² | ”°Ļõ”±ÖøµÄŹĒĻõĖį¼Ų |
| B | ·²Ä«ÉÕŃĢÄżÖŹ¶ųĪŖÖ® | ”°ŃĢ”±ÖøµÄŹĒ½¹Ģæ |
| C | ·²Ä«Ī±·½ŹæŅŌĀÆ»š»óČĖÕߣ¬ĪØÖģÉ°ŅųÓŽČĖŅ×»ó | ”°ÖģÉ°Ņų”±ÖøµÄŹĒAg2S |
| D | Ī彚֮³¤£¬ČŪ»Æ³ÉŠĪÖ®ŗó£¬×”ŹĄÓĄĪŽ±äøü | ÕāĆū»°ĆčŹöµÄ¶ŌĻóĪŖCu |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
| Ó¦³ĘČ”NaOHµÄÖŹĮæ/g | ӦєÓĆČŻĮæĘæµÄ¹ęøń/mL | ³żČŻĮæĘæĶā»¹ŠčŅŖµÄĘäĖü²£Į§ŅĒĘ÷ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | MgO | B£® | Al£ØOH£©3 | C£® | SiO2 | D£® | AlCl3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 1mol°ĀɳĄąŗ£Ø £© Óė×ćĮæNa2CO3ČÜŅŗ·“Ó¦ÄÜÉś³É4molC O2B £© Óė×ćĮæNa2CO3ČÜŅŗ·“Ó¦ÄÜÉś³É4molC O2B | |
| B£® | ŅŃÖŖ·“Ó¦mX£Øg£©+nY£Øg£©?qZ£Øg£©£¬ČōĘ½ŗāŹ±X”¢YµÄ×Ŗ»ÆĀŹĻąµČ£¬ĖµĆ÷·“Ó¦æŖŹ¼Ź±X”¢YµÄĪļÖŹµÄĮæÖ®±ČĪŖ1£ŗ1 | |
| C£® | 1L 1mol/LNa2CO3ČÜŅŗÖŠŗ¬ÓŠ3”Į6.02”Į1023øöĄė×Ó | |
| D£® | ŌŚŅ»¶ØĪĀ¶ČĻĀ£¬ĻąĶ¬pHµÄĮņĖįŗĶĮņĖįĢśČÜŅŗÖŠĖ®µēĄė³öĄ“µÄc£ØH+£©·Ö±šŹĒ1.0”Į10-amol•L-1ŗĶŹĒ1.0”Į10-bmol•L-1£¬ŌŚ“ĖĪĀ¶ČŹ±£¬Ė®µÄĄė×Ó»żĪŖ1.0”Į10-£Øb+a£© |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com