
| H+ |
| ת�� |
| Fe2+ |
| ��ԭ |
| OH- |
| ���� |
| 10 |
| 11 |
| A�������̷���FeSO4?7H2O����M=278������ԭ��������1L��ˮ��������Ҫ917.4g |
| B����Һ��ɫ���ֲ��䣬˵���������淴Ӧ�ﵽ��ƽ��״̬ |
| C��������ת����Ӧ��ƽ�ⳣ��K=1��1014����ת����������Һ��pH=6 |
| D��������Ksp[Cr��OH��3]=1��10-32��Ҫʹ�������ˮ��c��Cr3+������1��10-5mol/L��Ӧ����Һ��pH=5 |
| 10 |
| 11 |
| 10 |
| 11 |
| 28.6g |
| 52g/mol |
| 28.6g |
| 52g/mol |
| 10 |
| 11 |
| 10 |
| 11 |
| 10 |
| 11 |
| 1 |
| 2 |
| c(Cr 2O72-) |
| c2(CrO42-)c2(H+) |


 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������� |
| B����������ľ̿���� |
| C��NaCl����ˮ |
| D��HI�������ȷֽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��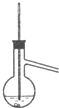 |
B��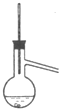 |
C��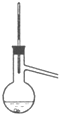 |
D��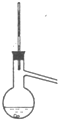 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��4HCl��Ũ��+MnO2
| ||||
| B��Zn+2HCl�TZnCl2+H2�� | ||||
C��Cu+2H2SO4��Ũ��
| ||||
| D��2Na2O2+4HCl�T4NaCl+O2��+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ƾ���ˮ |
| B�����ͺ�ˮ |
| C������Ȼ��Ƶ�ˮ��Һ |
| D����ɳ��ʳ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��KOH+CH3COOH=CH3COOK+H2O |
| B��Ba��OH��2+H2SO4=BaSO4��+2H2O |
| C��Cu��OH��2+H2SO4=CuSO4+2H2O |
| D��2NaOH+H2SO4=Na2SO4+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����������ɫ�����ӷ���Һ�� |
| B��ϡ����������Ա�Ũ����ǿ |
| C��Ũ������óʻ�ɫ |
| D��Ũ������������²������κη�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
2- 4 |
| H+ |
| ��ת�� |
2- 7 |
| Fe2+ |
| �ڻ�ԭ |
| OH- |
| �۳��� |
| A��������м��ᣬ����Һ��pH ������2����Һ�Ի�ɫ��CrO42-����Ũ������ |
| B��������е�2v��CrO42-��=v��Cr2O72-��ʱ��˵����Ӧ2CrO42-����ɫ��+2H+?Cr2O72-����ɫ��+H2O�ﵽƽ��״̬ |
| C��������У���Ҫ��ԭ1mol Cr2O72-���ӣ���Ҫ12mol ��NH4��2Fe��SO4��2?6H2O |
| D��������У�������Һ��pH������6 ʱ�������Ϊ��ˮ�еĸ��ѳ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com