
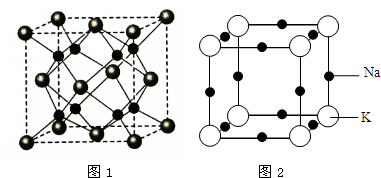
���� ��1���������ܹ��γɻ�����A��������λ�ھ����Ķ�������ģ�������λ�ھ��������ģ���Na�ĸ���Ϊ8��O�ĸ���Ϊ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4��N��Na����N��O��=2��1�����γɵĻ�����ΪNa2O�����ݾ����Ľṹ�ж���λ��������������������ɼ����ܶȣ�
��2������Ӳ��Ӵ�ģ�Ϳ�֪����Խ����ķ�֮һ����ԭ���붥���ϵ�ԭ�ӽ������辧���߳�Ϊx������$\frac{1}{4}$����$\sqrt{3}$x��=$\frac{a+b}{2}$����������ı߳���
��3�����ݾ�̯����֪����������ԭ����Ϊ12��$\frac{1}{4}$=3����ԭ����Ϊ8��$\frac{1}{8}$=1���ݴ�ȷ���Ͻ�Ļ�ѧʽ�����ݾ���ͼ��֪��ÿ��K ԭ����Χ��6����ԭ�ӣ����ݾ����Ľṹ��֪�������ı߳�Ϊ��ԭ�Ӻͼ�ԭ�ӵ�ֱ��֮�ͣ�����Ŀռ�������Ϊ$\frac{��ԭ�����ԭ�ӵ����֮��}{���������}$��100%���ݴ˴��⣮
��� �⣺��1���������ܹ��γɻ�����A��������λ�ھ����Ķ�������ģ�������λ�ھ��������ģ���Na�ĸ���Ϊ8��O�ĸ���Ϊ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4��N��Na����N��O��=2��1�����γɵĻ�����ΪNa2O��
������Oλ�����ģ�ÿ����������4��Na��O�ľ��������ÿ������Ϊ2���������У�����Oԭ�ӵ���λ��Ϊ8��
����������Ϊ$\frac{4��62g/mol}{6.02��1{0}^{23}/mol}$�����������Ϊ��0.566��10-7��cm3������F���ܶ�Ϊ$\frac{4��62g/mol}{��0.566��1{0}^{-7}cm��^{3}��6.02��10{\;}^{23}/mol}$��
�ʴ�Ϊ��Na2O��8��$\frac{4��62g/mol}{��0.566��1{0}^{-7}cm��^{3}��6.02��10{\;}^{23}/mol}$��
��2������Ӳ��Ӵ�ģ�Ϳ�֪����Խ����ķ�֮һ����ԭ���붥���ϵ�ԭ�ӽ������辧���߳�Ϊx������$\frac{1}{4}$����$\sqrt{3}$x��=$\frac{a+b}{2}$����ã�x=$\frac{2\sqrt{3}}{3}$��a+b����
�ʴ�Ϊ��$\frac{2\sqrt{3}}{3}$��a+b����
��3�����ݾ�̯����֪����������ԭ����Ϊ12��$\frac{1}{4}$=3����ԭ����Ϊ8��$\frac{1}{8}$=1�����ԺϽ�Ļ�ѧʽΪKNa3��
���ݾ���ͼ��֪��ÿ��K ԭ����Χ��6����ԭ�ӣ����Ծ�����K ԭ�ӵ���λ��Ϊ6����������ԭ�Ӻͼ�ԭ�����֮��Ϊ$\frac{4}{3}$�У�1863��3+2273�������ݾ����Ľṹ��֪�������ı߳�Ϊ��ԭ�Ӻͼ�ԭ�ӵ�ֱ��֮��Ϊ2����186pm+227pm�������Ծ��������Ϊ��2��186pm+2��227pm��3������Ŀռ�������Ϊ$\frac{��ԭ�����ԭ�ӵ����֮��}{���������}$��100%=$\frac{\frac{4}{3}�У�18{6}^{3��}3+22{7}^{3}��}{��186��2+227��2��^{3}}$��100%��
�ʴ�Ϊ��KNa3��6��$\frac{\frac{4}{3}�У�18{6}^{3��}3+22{7}^{3}��}{��186��2+227��2��^{3}}$��100%��
���� ������Ҫ���龧�����㡢�ռ������ʵļ���ȣ���Ҫѧ������һ���Ŀռ���������ѧ������������Ŀ�Ѷ��еȣ�


 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д� ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ݢ� | B�� | �ڢܢݢޢ� | C�� | �٢ۢݢ� | D�� | �ڢܢޢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ϵͳ���������л��CH3��2CHCH��CH2CH3����CH2CH2CH3��������Ϊ��2-��-3-�һ����� | |
| B�� | Ӳ֬��������������������Ϊͬϵ�� | |
| C�� | �������ױ��ķ���ʽΪC7H5N3O6 | |
| D�� | ��������ά�صĻ�ѧʽΪ��C6H10O5��n�������߲���ͬ���칹�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ԭ��ص�������п | B�� | ͭ�缫������ԭ��Ӧ | ||
| C�� | ԭ�����Cu2+��п���ƶ� | D�� | ͭ��������ų� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ۢ� | B�� | �ܢ� | C�� | �ܢޢ� | D�� | �٢ߢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ӧ������I2��Ũ���й� | |
| B�� | HI�ڷ�Ӧ�������������� | |
| C�� | ��Ӧ�ʱ�ľ���ֵ����190kJ•mol-1 | |
| D�� | ��ȩ�ķ�Ӧ������Ҫȡ���ڷ�Ӧ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | V=896 | B�� | ��ҺA�е�������ΪFe2+��Fe3+��H+ | ||
| C�� | ��Ʒ��CuO������Ϊ4.0g | D�� | ��Ʒ��FeԪ�ص�����Ϊ2.24g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
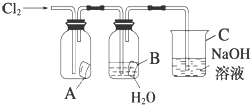 ijͬѧ��ͼ����ʵ�飬��A�зŵ��Ǹ���ĺ�ɫֽ����B�зŵ���ʪ��ĺ�ɫֽ����C��ʢ�ŵ�������������Һ����ش��������⣮
ijͬѧ��ͼ����ʵ�飬��A�зŵ��Ǹ���ĺ�ɫֽ����B�зŵ���ʪ��ĺ�ɫֽ����C��ʢ�ŵ�������������Һ����ش��������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
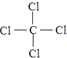 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com