
��֪2RCH2CHO 
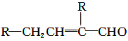
ˮ������EΪ���������ռ������������Ʒ�ɹ˪��E��һ�ֺϳ�·�����£�
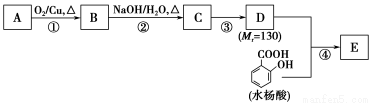
��ش��������⣺
(1)һԪ��A��������������ԼΪ21.6%����A�ķ���ʽΪ________���ṹ������ʾAֻ��һ������A������Ϊ________��
(2)B�������Ƶ�Cu(OH)2������Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ____________________________��
(3)C��________�ֽṹ����һ��ȡ��������C�����������ţ���ʹ�õ��Ⱥ�˳��д�������Լ���______________________________________________��
(4)�������ķ�Ӧ����Ϊ________��D���������ŵ�����Ϊ________��
(5)д��ͬʱ��������������ˮ��������ͬ���칹��Ľṹ��ʽ��_____________________________��
a����������6��̼ԭ����һ��ֱ���ϣ�
b�����������������Ű���ˮ������еĹ����š�
(6)�������ķ�Ӧ����Ϊ______________��д��E�Ľṹ��ʽ��________________��
(1)C4H10O
1?����(��������)
(2)CH3CH2CH2CHO��2Cu(OH)2��NaOH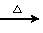 CH3CH2CH2COONa��Cu2O����3H2O
CH3CH2CH2COONa��Cu2O����3H2O
(3)2��������Һ��ϡ���ᡢ��ˮ(������������)
(4)��ԭ��Ӧ(��ӳɷ�Ӧ)���ǻ�
(5) 

(6)ŨH2SO4������

�������������ṩ��Ϣ��ת������ȷ����Ӧ�P��Ӧ���͡�
(1)AΪһԪ��������������������ԼΪ21.6%��������Է�������Mr��16��21.6%��74�������ʽΪCxHyO����12x��y��16��74,12x��y��58����x��4ʱ��y��10������ʽΪC4H10O����x��3��x��5ʱ��������������A�ķ���ʽΪC4H10O��A��ֻ��һ����������AΪ
CH3CH2CH2CH2OH������Ϊ1?����(��������)��
(2)����������֪BΪCH3CH2CH2CHO��B�����Ƶ�Cu(OH)2�ķ�ӦΪCH3CH2CH2CHO��2Cu(OH)2��NaOH CH3CH2CH2COONa��Cu2O����3H2O��
CH3CH2CH2COONa��Cu2O����3H2O��
(3)������ṩ����Ϣ��֪���ķ�Ӧ����Ϊ
2CH3CH2CH2CHO

����C��2�ֽṹ����CHO�� ��ͬ����ʱҪ�ȼ�����CHO��Ȼ�����
��ͬ����ʱҪ�ȼ�����CHO��Ȼ����� �������Լ�Ϊ������Һ��ϡ�������ˮ��
�������Լ�Ϊ������Һ��ϡ�������ˮ��
(4)C����Է�������Ϊ126��D����Է�������Ϊ130������C�D��D����H2�����ӳɷ�Ӧ��Ҳ��Ϊ��ԭ��Ӧ��������Է����������4������ ����CHO����H2�ӳɣ�����D�й���������Ϊ�ǻ���
����CHO����H2�ӳɣ�����D�й���������Ϊ�ǻ���
(5)  �IJ����Ͷ�Ϊ5������ͬ���칹�����6��̼ԭ����һ��ֱ���ϣ�ͬʱ��������COOH����OH��������̼��������������˫������COOHΪһ�������Ͷȣ���������2��������4��˫��������Ҫ��6��̼ԭ����һ��ֱ���ϣ�����Ҫ�������C��C��C��C�������Ľṹ��������������COOH����OH����CH3��CH����COOH����OH��2����CH2�������Կ��ܵĽṹ�У�
�IJ����Ͷ�Ϊ5������ͬ���칹�����6��̼ԭ����һ��ֱ���ϣ�ͬʱ��������COOH����OH��������̼��������������˫������COOHΪһ�������Ͷȣ���������2��������4��˫��������Ҫ��6��̼ԭ����һ��ֱ���ϣ�����Ҫ�������C��C��C��C�������Ľṹ��������������COOH����OH����CH3��CH����COOH����OH��2����CH2�������Կ��ܵĽṹ�У�
 ��
��
(6)������Ϊ������Ӧ������������ŨH2SO4�����ȣ�E�Ľṹ��ʽΪ
 ��
��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ����ר��ͻ�� ר����������ԭ��Ӧ��ϰ���������棩 ���ͣ�ѡ����
���ữ����MnSO4��Һ�еμ�(NH4)2S2O8(���������)��Һ�ᷢ����Ӧ��Mn2����S2O82-��H2O�D��MnO4-��H����SO42-������˵������ȷ����(����)
A���������ø÷�Ӧ����Mn2��
B�������ԱȽϣ�S2O82-��MnO4-
C��MnSO4��Һ����ʹ�������ữ
D������0.1 mol�����������ɣ���ת�Ƶ���0.5 mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ����ר��ͻ�� ר��ʮ���ۺ�ʵ��̽����ϰ���������棩 ���ͣ������
���÷Ͼɶ�п��Ƥ���Ʊ�����Fe3O4�������Ӽ�������ZnO���Ʊ�����ͼ���£�
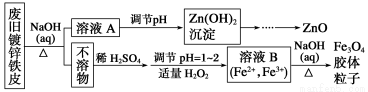
��֪��Zn���仯�����������Al���仯������������ƣ���ش��������⣺
(1)��NaOH��Һ�����Ͼɶ�п��Ƥ��������________��
A��ȥ������ B���ܽ��п�� C��ȥ������ D���ۻ�
(2)������ҺA��pH�ɲ���Zn(OH)2������Ϊ�Ƶ�ZnO����������������________��
(3)����ҺB��ȡFe3O4�������ӵĹ����У������ͨ��N2����ԭ����
_______________________________________________________________
(4)Fe3O4���������ܷ��ü�ѹ���˷�ʵ�ֹ�Һ���룿__________(������������������)��������________________________________��
(5)���ظ���ط�(һ��������ԭ�ζ���)�ɲⶨ����Fe3O4�еĶ�������������������Ũ��Ϊ0.010 00 mol��L��1��K2Cr2O7����Һ250 mL��Ӧȷ��ȡ________ g K2Cr2O7(����4λ��Ч���֣���֪MK2Cr2O7��294.0 g��mol��1)�����Ƹñ���Һʱ�����������в���Ҫ�õ�����________(�ñ�ű�ʾ)��
��������ƽ�����ձ�������Ͳ����������
������ƿ ����ͷ�ιܡ�����Һ��
(6) �ζ������У�����ζ�ǰװ��K2Cr2O7����Һ�ĵζ��ܼ��첿�������ݣ����ζ�������������ʧ����ⶨ�����________(����ƫ��������ƫС������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ����ר��ͻ�� ר��ʮ������ѧ�ۺ�Ӧ����ϰ���������棩 ���ͣ������
�ⱻ��Ϊ������Ԫ��������ѧ�����ز����ɷ��ε�ȱ������KI��KIO3���Ⱥ����ڼӵ����С�
(1)��ҵ�Ͽ���ͨ����м������KI���乤���������£�
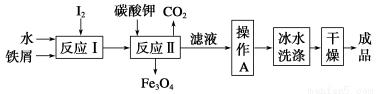
����Ӧ�����������Ļ�������û���������Ԫ�����Ԫ�ص�������Ϊ21��127�����������̼���ʱ����Ӧ���Ļ�ѧ����ʽΪ____________________��
������A����__________________���ñ�ˮϴ�ӵ�Ŀ����__________________��
(2)KIO3����ͨ��H2O2����I2���Ƶ�HIO3��Ȼ������KOH�к͵ķ�������������
�����ʱ����KIO3��ʳ�γ��ڳ���ǰ���룬��ԭ����_____________________________
�����Ƶ�1 284 kg KIO3���壬������������������������Ϊ30%��˫��ˮ________ kg��
��KIO3����ͨ����ͼ��ʾԭ�������Ʊ������ʱ�ܷ�Ӧ�����ӷ���ʽΪ____________����������Һ����ı仯�������������������pH����ǰ���________(ѡ����������������С������������)��
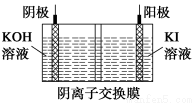
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ����ר��ͻ�� ר��ʮ���л���ѧ������ϰ���������棩 ���ͣ������
�һ����ȩ(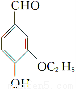 )��ʳƷ���Ӽ�������ԭ�ϡ�
)��ʳƷ���Ӽ�������ԭ�ϡ�
(1)д���һ����ȩ�������������ֺ��������ŵ�����______________________��
(2)���һ����ȩ��Ϊͬ���칹�壬����NaHCO3��Һ��Ӧ�ų����壬�ұ�����ֻ��һ������(����R��O��R����R��O��COOH�ṹ)����________�֡�
(3)A������ͬ���칹���е�һ�֣��ɷ������±仯��
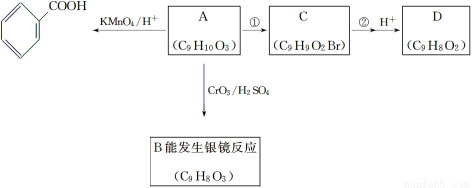
��֪����.RCH2OH 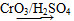 RCHO��
RCHO��
��.�뱽��ֱ��������̼ԭ��������ʱ����̼ԭ�Ӳſɱ�����KMnO4��Һ����Ϊ�Ȼ���
a��д��A�Ľṹ��ʽ��___________________________��
��Ӧ��������������______________________________��
b����A��ֱ������D����Ӧ��������Ŀ����_________________________��
c��д����Ӧ����ʽ��A��B______________________________��
C��NaOHˮ��Һ���ȣ�___________________________________��
(4)�һ����ȩ����һ��ͬ���칹��E
( )��һ��ҽҩ�м��塣������ȩ(
)��һ��ҽҩ�м��塣������ȩ( )�ϳ�E(����ԭ����ѡ)���漰�ķ�Ӧ������(����Ӧ˳����д)________________________��
)�ϳ�E(����ԭ����ѡ)���漰�ķ�Ӧ������(����Ӧ˳����д)________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ����ר��ͻ�� ר��ʮһ��������Ԫ����ϰ���������棩 ���ͣ�ѡ����
�����仯����������������й㷺Ӧ�á���ش��������⣺
(1)������(FeS2)�����������ұ����������Ҫԭ�ϡ�����һ����ӦΪ3FeS2��8O2 6SO2��Fe3O4����3 mol FeS2�μӷ�Ӧ��ת��________ mol���ӡ�
6SO2��Fe3O4����3 mol FeS2�μӷ�Ӧ��ת��________ mol���ӡ�
(2)�Ȼ�����Һ������ӡˢ��·ͭ�帯ʴ������Ӧ�����ӷ���ʽΪ________________���Ӹ�ʴ��Һ���յõ�����ͭ������Ҫ���Լ���__________________________��
(3)���������ƣ�������Ҳ������ˮ������ʹ��ʱ����������������ʹ���Է�ˮ�е������������ȥ����ԭ����____________________________
(4)�����ĵ绯ѧ��ʴԭ����ͼ��ʾ������ͼ�����ļ��ɳ�Ϊ�����绯ѧ�����ļ�ʾ��ͼ������ͼ�����߿��������ģ����ü�ͷ���������������
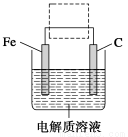
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ����ר��ͻ�� ר��ʮһ��������Ԫ����ϰ���������棩 ���ͣ�ѡ����
ijͬѧ��Na2O2��CO2��Ӧ������ù�������о�������ʵ�鷽���в��ܲ�ù�����Na2CO3��������������(����)
A��ȡa�˻������������ϡ�����ַ�Ӧ������Ӧ��Ĺ���������ɡ����յõ�b�˹���
B��ȡa�˻������������ϡ�����ַ�Ӧ���ݳ������ü�ʯ�����գ�����b��
C��ȡa�˻������������BaCl2��Һ��ַ�Ӧ�����ˡ�ϴ�ӡ���ɵõ�b�˹���
D��ȡa�˻������������ˮ��Ӧ�����ȣ�ʹ������ȫ�ݳ�����ȴ�����²���������ΪV L(���)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ����ר��ͻ�� ר�������ʽṹ��Ԫ����������ϰ���������棩 ���ͣ�ѡ����
�ݹ���ý�屨���������ǿ쳵���ź������ǿ쳵����̽�����ֱ��ڻ��Ǻͽ��Ǵ������з�����һ�ַdz��������̬��������ֻ�����Ĵ��ڲ����ᵼ�½����ϵ�����ЧӦ���ɱ��ķŴ��ҿ��ܻ��ڻ�����Ҳ�շ�����ЧӦ�IJ��������ĽṹʽΪ16O=C=18O������˵����ȷ���ǣ� ��
A��16O��18OΪͬ��ԭ��
B��16O=C=18O��16O=C=16O��Ϊͬλ��
C��16O=C=18O��16O=C=16O�Ļ�ѧ���ʼ�����ͬ
D��16O=C=18O��Na216O2��Ӧ���ɵ������к�18O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ����ר��ͻ�� ר��Ż�ѧ��Ӧ���ʻ�ѧƽ����ϰ���������棩 ���ͣ������
��̼����(��Ҫָ����CO2)�ڽ������������ŷ��о�����Ҫ�����á�ĿǰNH3��(NH4)2CO3�Ѿ���������ҵ��̼����������CO2�ɷ������¿��淴Ӧ��
��Ӧ����2NH3(l)��H2O(l)��CO2(g)  (NH4)2CO3(aq)��H1
(NH4)2CO3(aq)��H1
��Ӧ����NH3(l)��H2O(l)��CO2(g)  NH4HCO3(aq)��H2
NH4HCO3(aq)��H2
��Ӧ����(NH4)2CO3(aq)��H2O(l)��CO2(g)  2NH4HCO3(aq)��H3
2NH4HCO3(aq)��H3
��ش��������⣺
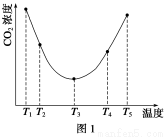
��2��Ϊ�о��¶ȶ�(NH4)2CO3����CO2Ч�ʵ�Ӱ�죬��ij�¶�T1�£���һ������(NH4)2CO3��Һ�����ܱ������У�������һ������CO2����(�õ�����Ϊϡ�ͼ�)����tʱ�̣����������CO2�����Ũ�ȡ�Ȼ��ֱ����¶�ΪT2��T3��T4��T5�£�����������ʼʵ���������䣬�ظ�����ʵ�飬������ͬʱ����CO2����Ũ�ȣ��õ�����ͼ(��ͼ1)����
����H3________0(������������������������)��
����T1��T2��T4��T5�����¶����䣬������CO2����Ũ�ȳ�����ͼ1��ʾ�ı仯���ƣ���ԭ����____________________________________________________________________________��
����Ӧ�����¶�ΪT1ʱ����ҺpH��ʱ��仯������������ͼ2��ʾ����ʱ�䵽��t1ʱ�����÷�Ӧ��ϵ�¶�Ѹ��������T2����ά�ָ��¶ȡ����ڸ�ͼ�л���t1ʱ�̺���Һ��pH�仯���������ߡ�
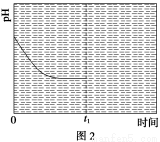
��3�����÷�Ӧ������CO2����(NH4)2CO3��ʼŨ�Ⱥ����ȷ��������£����CO2�������Ĵ�ʩ��________________________(д��2��)��
��4������������Ҳ������ΪCO2���������__________��
A��NH4Cl B��Na2CO3
C��HOCH2CH2OH D��HOCH2CH2NH2
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com