
��ϩ��������������������Ʊ����ϡ���֬�ȸ߾������Ҫ�м��壬��������·�ߺϳɣ�
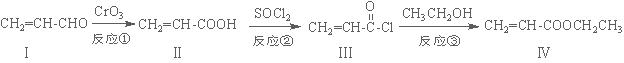 ��1����������ķ���ʽΪ ��1 mol���������ȫȼ������O2Ϊ mol��
��1����������ķ���ʽΪ ��1 mol���������ȫȼ������O2Ϊ mol��
��2�����������ʹ��ˮ��ɫ���䷴Ӧ����ʽΪ ��
��3����Ӧ������ ��Ӧ�������������ɻ������������ʽΪC3H6O���������õ������������ķ�Ӧ����ʽΪ ��
��4����������ǻ��������ͬ���칹�壬������̼̼˫��������NaHCO3��Һ��Ӧ�ų����壬��˴Ź����������֮��Ϊ1��1��6��������Ľṹ��ʽΪ ��
��5��һ�������£�������  Ҳ���뻯����������Ʒ�Ӧ�۵ķ�Ӧ����õ��IJ���Ľṹ��ʽΪ ��
Ҳ���뻯����������Ʒ�Ӧ�۵ķ�Ӧ����õ��IJ���Ľṹ��ʽΪ ��
��֪ʶ�㡿�л���Ľṹ������ L2 L4 L7
���𰸽�����(1)C5H8O2 �� 6��
(2)CH2=CH-COOH+Br2��CH2Br-CHBr-COOH
(3)ȡ�� 2CH2=CH-CH2OH+O2 2CH2=CH-CHO+2H2O
2CH2=CH-CHO+2H2O
(4)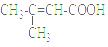 (5)
(5)
��������1�����������һ��������5��̼ԭ�ӡ�8����ԭ�ӡ�2����ԭ�ӣ�����ʽΪC5H8O2��1 mol���������ȫȼ������O2Ϊ����5+2-1��=6mol��
��2���������ķ��ӽṹ�к�̼̼˫��������ˮ�����ӳɷ�Ӧ���䷴Ӧ����ʽ��CH2=CH-COOH+Br2��CH2Br-CHBr-COOH
��3�����������ӽṹ�е��ǻ�����ԭ��ȡ���������������ɻ������������ʽΪC3H6O���������õ��������Ϊ���࣬�ṹ��ʽ��CH2=CH-CH2OH����Ӧ����ʽΪ�� 2CH2=CH-CH2OH+O2 2CH2=CH-CHO+2H2O
2CH2=CH-CHO+2H2O
��4����������ǻ��������ͬ���칹�壬������NaHCO3��Һ��Ӧ�ų�����õ������Ȼ�����˴Ź����������֮��Ϊ1��1��6��������Ľṹ��ʽΪ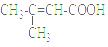
��ȷ�Ӧ�ۣ����ȡ����Ӧ���ص㼴�ɵõ�����Ľṹ��ʽ��
��˼·�㲦�����⿼�����л�������ż��ת�����Ƚϻ�����


 �Ƹ�С״Ԫͬ������������ϵ�д�
�Ƹ�С״Ԫͬ������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�±����������У�����֮��������ʵ����ͼ��ʾת������
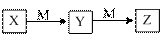
| ѡ�� | X | Y | Z | M |
| A | NH3 | NO | NO2 | O2 |
| B | Cl2 | FeCl3 | FeCl2 | Fe |
| C | Al | Al(OH)3 | NaAlO2 | NaOH |
| D | NaOH | Na2CO3 | NaHCO3 | CO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͭ���Ʊ�Cu—Zn—Alϵ��������Ҫԭ�ϣ���ȡ����ͭ����������Ʒ����ɹ�ѡ�ã�
��Cu��ϡ���ᷴӦ��ȡ��3Cu��8HNO3(ϡ)===3Cu(NO3)2��2NO����4H2O
��Cu��Ũ���ᷴӦ��ȡ��Cu��4HNO3(Ũ)===Cu(NO3)2��2NO2����2H2O
��ͨ������ͭм��ϡ�������ϵ����ȡ��2Cu��4HNO3��O2===2Cu(NO3)2��2H2O
����˵����ȷ���� (����)
A����ȡ��ͬ��������ͭ��������������
B����ȡ��ͬ��������ͭ�ٲ������ж�����Ȣڶ�
C�����ַ���������������ʢ�>��>��
D�����ַ����ķ�Ӧ�����������������н���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijѧϰС������ͼװ�òⶨþ���Ͻ������������������������ԭ��������
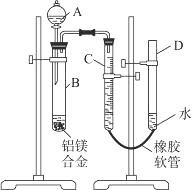
��1��A���Լ�Ϊ______________��
��2��ʵ��ǰ���Ƚ���þ�Ͻ���ϡ���н���Ƭ�̣���Ŀ����____________________________��
��3����������ԣ���ҩƷ��ˮװ��������У���
�Ӻ�װ�ú�����еIJ������У��ټ�¼C��Һ��
λ�ã��ڽ�B��ʣ�������ˡ�ϴ�ӡ�������أ�
�۴�B�в���������������ָ������º�¼C��Һ��λ�ã�
����A��B�еμ������Լ���
����������˳����___________������ţ����ڽ��в�����ʱ��
¼C��Һ��λ��ʱ��������ƽ���⣬��Ӧ_ ��
��4��B�з�����Ӧ�Ļ�ѧ����ʽΪ_________________________________________________��
��5����ʵ������þ�Ͻ������Ϊa g������������Ϊb mL���ѻ���Ϊ��״������B��ʣ����������Ϊc g�����������ԭ������Ϊ_______________��
��6��ʵ������У���δϴ�ӹ������õIJ����������������������________���ƫ����ƫС��������Ӱ�족����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ȤС�����HgSO4��������ʹ��Ȳˮ��Ϊ��ȩ����75�����ң�����HgSO4����ijЩ�ض����ʳ��ᷢ�������ж���ʧȥ�����ã�H2S��������һ�֡���ѡ�ÿ�״��ʯ��ŨH2SO4��ˮ��NaOH��Һ��HgO��ĩ������������ȩ��װ��ͼ������ʾ���ش��������⣺
��1��ʵ�鿪ʼʱ������A��ʢ�ŵ�ʯ��B��Ӧװ��_______����������_________ ��
��2������D��ʢ��ˮ����������__________________________________________ ��
��3��������ƿF��Ӧ����HgO�����������Լ����������߷ֱ�ֱ�Ӽ��룬�밴������Ⱥ�˳��д������HgO���ڵĸ����Լ�������_______________________ ��
��4����ѡ���¶ȼ�G�����̱�ʾ��ȷ����________ ��
A��0�桫50�� B��0�桫100�� C��0�桫200�� D��50�桫100��
��5��������ȩ���Ƴ��IJ�����������______________________________________ ��
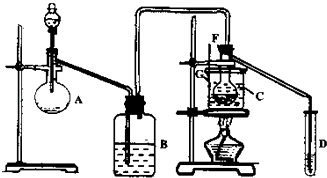
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ���ǣ� ��
A����һ�������£���ϩ����H2�����ӳɷ�Ӧ����������H2�����ӳɷ�Ӧ
B��C2H6O��C4H10����2��ͬ���칹��
C���������������������NaOH��Ӧ�����߷����й�������ͬ
D�����ۺ͵����ʾ���ˮ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ӻ�ˮ����ȡþ�Ĺ�������ͼ�ɱ�ʾ���£�
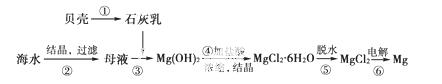
����˵������ȷ��������
A���ô˷���ȡþ���ŵ�֮һ��ԭ����Դ�ḻ
B��������MgCl2ʱ������������
C������ݣ��ɽ���������HCl�����Χ����ˮ
D�����������������漰�����ϡ��ֽ���ֽⷴӦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������������ֱ�Ͷ�뵽������������ʵ���Ũ�ȵ����ᡢ����������Һ�У�����H2�����֮����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڳ����£���pH��8��NaOH��Һ��pH��10��NaOH��Һ�������Ϻ���Һ��pH��ӽ���(����)
A��8.3 B��8.7 C��9 D��9.7
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com