
| ʱ��/min | C0 | Zn | CO2 |
| 0 | 0.11 | 0 | 0 |
| 2 | 0.1 | 0.01 | 0.01 |
| 3 | 0.01 | 0.1 | 0.1 |
| 4 | 0.01 | 0.1 | 0.1 |
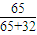 =
=
 =41.5%
=41.5% ×100%=14.3%��ʵ��ͨ��Ŀ���Ҫ�࣬��SO2���������������14.3%��
×100%=14.3%��ʵ��ͨ��Ŀ���Ҫ�࣬��SO2���������������14.3%�� ×100%=90.9%��
×100%=90.9%�� =1�����m=12.25
=1�����m=12.25

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʱ��/min | CO | Zn | CO2 |
| 0 | 0.11 | 0 | 0 |
| 2 | 0.10 | 0.01 | 0.01 |
| 3 | 0.01 | 0.10 | 0.10 |
| 4 | 0.01 | 0.10 | 0.10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʱ��/min | C0 | Zn | CO2 |
| 0 | 0.11 | 0 | 0 |
| 2 | 0.1 | 0.01 | 0.01 |
| 3 | 0.01 | 0.1 | 0.1 |
| 4 | 0.01 | 0.1 | 0.1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ�����и�����һ���ʼ컯ѧ�Ծ��������棩 ���ͣ������
��l������п�ǽ���п����Ҫ�ɷ���ZnS��ͨ����ѡ����ʹ��ת��Ϊ����п���ٰ�����п�ͽ� ̿��ϣ��ڹķ�¯�м��ȵ�1100~1300�棬ʹп���������
��д������п����Ҫ��Ӧ��
���շ�Ӧ�� ��
�ķ�¯�п��ܷ����ķ�Ӧ�� ����дһ����
�ڴӱ��������ͳ������ԭ�ϽǶȿ���δ��������ò�����������
��2����ҵ��ұ�������ǵ��������
��ұ�����ĵ����е��������������Ͼ���ʯ�����ƺ�ú�ĸ����Ʒ �����������ƣ�
�����������۵�ܸߣ�������ұ����Ҫ�������ʯ��Na3AlF������������____ ��
�۹�ҵ��ұ����ʱ�õ�ԭ����Al2O3��������AlCl3����ԭ���� ��
��3����ҵ�ϡ������Ƽ������Ҫ��Ӧ�Ļ�ѧ����ʽ�� �����е�CO2��Դ�� ��
��4����������Ʋ�����ԭ��֮һ����ҵ���Ʋ������ڲ�����¯�н��У����з�Ӧ֮һΪ��
 ���������������£���l000ag CaCO3��60ag
SiO2��ϣ������ɵ�CO2�ڱ�״���µ����Ϊ ���ú�a�Ĵ���ʽ��ʾ����
���������������£���l000ag CaCO3��60ag
SiO2��ϣ������ɵ�CO2�ڱ�״���µ����Ϊ ���ú�a�Ĵ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ���Ϻ���¬����������ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
����п�ǽ���п����Ҫ��ZnS������ʹ��ת��Ϊ����п���ٰ�����п�ͽ�̿��ϣ��ڹķ�¯�м�����1100�桫1300�棬ʹп����������������N2��O2����������ֱ�Ϊ0.8��0.20������Ҫ��ӦΪ��
����¯�У�2ZnS(s) + 3O2(g) �� 2ZnO(s) + 2SO2(g) ��
�ķ�¯�У�2C(s) + O2(g) ��2CO ��
�ķ�¯�У�ZnO(s) + CO(g) Zn(g)+CO2(g)
��
Zn(g)+CO2(g)
��
��1����֪��п���к���16��(�������ʲ�����)������п������п�ĺ���Ϊ ��
��2������¯������¯����SO2��������������� %������С����һλ���֣���
�ǹķ�¯�ݻ��̶���¯�ڲ�����̬���������ʵ���Ũ�ȣ�mol/L���仯���£�
|
ʱ��/min |
CO |
Zn |
CO2 |
|
0 |
0.11 |
0 |
0 |
|
2 |
0.10 |
0.01 |
0.01 |
|
3 |
0.01 |
0.10 |
0.10 |
|
4 |
0.01 |
0.10 |
0.10 |
��ķ�¯��CO�ܵ�ת����Ϊ ����������CO�ܵ�������Ϊ95%����ÿ����1molZn��������Ҫ���佹̿ g��
����ZnSȫ��ת��ΪZn������¯������N2��O2��SO2���������N2ռ82.5%���ķ�¯��CO��ת����Ϊ62.5%����O2��ʣ�࣬��ÿ����1mol Zn��Ӧ����¯�ķ�¯�й������ʿ����� L(S.T.P)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com