
����ʵ���ͼ���Ľ��ۻ������ȷ����
| ʵ���ͼ�� | ���ۻ���� | |
| A | �����ھƾ��ƻ����ϼ����ۻ��������� | �����������ɵ���������и��۵� |
| B | ijCH3COOH��CH3COONa��ɵ�������Һ�У�����һ������ˮ | c(CH3COO-)һ������c(Na+)��c(NH4+)֮�� |
| C |
| �÷�Ӧ�Ȼ�ѧ����ʽΪ2CO(g)+O2(g)=2CO2(g)��H=-566 kJ/mol |
| D | ��������������ʢװ���Ũ���� | ����Ũ�����Ӧ |
 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2011?������һģ������ʵ���ͼ���Ľ��ۻ������ȷ���ǣ�������
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�걱�������ظ�����һ��ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ�ѡ����
����ʵ���ͼ���Ľ��ۻ������ȷ����
|
|
ʵ���ͼ�� |
���ۻ���� |
|
A |
�����ھƾ��ƻ����ϼ����ۻ��������� |
�����������ɵ���������и��۵� |
|
B |
ijCH3COOH��CH3COONa��ɵ�������Һ�У�����һ������ˮ |
c(CH3COO-)һ������c(Na+)��c(NH4+)֮�� |
|
C |
|
�÷�Ӧ�Ȼ�ѧ����ʽΪ2CO(g)+O2(g)=2CO2(g)��H=-566 kJ/mol |
|
D |
��������������ʢװ���Ũ���� |
����Ũ�����Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������һģ ���ͣ���ѡ��
| ʵ���ͼ�� | ���ۻ���� | |
| A | �����ھƾ��ƻ����ϼ����ۻ��������� | �����������ɵ���������и��۵� |
| B | ijCH3COOH��CH3COONa��ɵ�������Һ�У�����һ������ˮ | c��CH3COO-��һ������c��Na+����c��NH4+��֮�� |
| C | �÷�Ӧ�Ȼ�ѧ����ʽΪ2CO��g��+O2��g��=2CO2��g����H=-566kJ/mol |
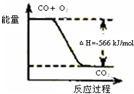 |
| D | ��������������ʢװ���Ũ���� | ����Ũ�����Ӧ |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�걱���������ظ߿���ѧһģ�Ծ��������棩 ���ͣ�ѡ����
| ʵ���ͼ�� | ���ۻ���� | |
| A | �����ھƾ��ƻ����ϼ����ۻ��������� | �����������ɵ���������и��۵� |
| B | ijCH3COOH��CH3COONa��ɵ�������Һ�У�����һ������ˮ | c��CH3COO-��һ������c��Na+����c��NH4+��֮�� |
| C | �÷�Ӧ�Ȼ�ѧ����ʽΪ2CO��g��+O2��g��=2CO2��g����H=-566kJ/mol | 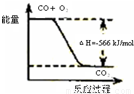 |
| D | ��������������ʢװ���Ũ���� | ����Ũ�����Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ���ͼ���Ľ��ۻ������ȷ����
|
| ʵ���ͼ�� | ���ۻ���� |
| A | �����ھƾ��ƻ����ϼ����ۻ��������� | �����������ɵ���������и��۵� |
| B | ijCH3COOH��CH3COONa��ɵ�������Һ�У�����һ������ˮ | c(CH3COO-)һ������c(Na+)��c(NH4+)֮�� |
| C | | �÷�Ӧ�Ȼ�ѧ����ʽΪ2CO(g)+O2(g)=2CO2(g)��H=-566 kJ/mol |
| D | ��������������ʢװ���Ũ���� | ����Ũ�����Ӧ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com