
������Щ��ʵ��ʵ����˵�������ԣ�Cl2>Br2>I2_____________________________________________________________________
(�����)��
����ˮ�ֱ����KBr��NaI��Һ����ɫ�������ˮ����NaCl��Һ�������Ա仯������KI������Һ�У���Һ����
��H2��Cl2�Ļ��������ձ�ը��H2��Br2�Ļ��������Ȳ��ܷ�Ӧ����H2��I2��Ӧ������
��Fe�ֱ���Cl2��Br2��I2��Ӧ����Fe�Ļ�����Ļ��ϼ۸ߵ�
��HCl��HBr��HI�����ȶ���Խ��Խ��
��Cl2��Br2��I2��ˮ�е��ܽ����С
 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��25 �桢101 kPa�£�1 g�״�ȼ������CO2��Һ̬ˮʱ����22.68 kJ�������Ȼ�ѧ����ʽ��ȷ����(����)
A��CH3OH(l)��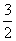 O2(g)===CO2(g)��2H2O(l)
O2(g)===CO2(g)��2H2O(l)
����H��725.76 kJ·mol��1
B��2CH3OH(l)��3O2(g)===2CO2(g)��4H2O(l)
����H����1 451.52 kJ·mol��1
C��2CH3OH(l)��3O2(g)===2CO2(g)��4H2O(l)
����H����725.76 kJ·mol��1
D��2CH3OH(l)��3O2(g)===2CO2(g)��4H2O(l)
����H��1 451.52 kJ·mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ȷ��ʾ���з�Ӧ�����ӷ���ʽΪ (����)��
A��̼��������Һ�е�������������Һ��HCO ��OH��===CO
��OH��===CO ��H2O
��H2O
B����������ͨ�����������Һ��SO2��ClO����OH��===SO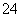 ��Cl����H2O
��Cl����H2O
C��������ϡ���BaS��2H��===H2S����Ba2��
D�����Ƶ�����������������������Һ��Al2O3��2OH����H2O===
2[Al(OH)4]��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ҫ����ա�
(1)��S2����Fe2����Fe3����Mg2����S��I����H���У�ֻ�������Ե���________��ֻ�л�ԭ�Ե���________���������������л�ԭ�Ե���________��
(2)ijͬѧд������������ѧ����ʽ(δ��ƽ)
��NO��HNO3����N2O3��H2O
��NH3��NO����HNO2��H2O
��N2O4��H2O����HNO3��HNO2
��������Ϊһ��������ʵ�ֵ���________��
(3)��������������ԭ��Ӧ�У���������ǿ��������______��
��2FeCl3��2KI===2FeCl2��2KCl��I2
��2FeCl2��Cl2===2FeCl3
��2KMnO4��16HCl(Ũ)===2KCl��2MnCl2��5Cl2����8H2O
��������Cl����I�����棬Ϊ������I����Cl�������������������⣬��Ӧ��������Ӧ�е�________����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ˮ��ʯ��ʯ�ķ�Ӧ����ȡ��Ũ��HClO��Һ�ķ���֮һ��ijͬѧ������һ������������ȡHClO��Һ�����������¶���ʵ�飺
�����Թ��м�������Ŀ�״̼��ƣ��ټ���Լ20 mL������ˮ����ַ�Ӧ�����������ݲ�������Һ�Ļ���ɫ��ȥ��
�ڹ��ˣ�����Һ������ɫ�����ϣ�������Ư���Ը�ǿ��
��Ϊ��ȷ����Ӧ�������Һ��Ϊ���ݣ��ֱ��������ʵ�飺
��һ����ʯ��ˮ��ϣ���������������ɫ������
�ڶ�����ϡ�����ϣ����̲����������ݣ�
�����ݼ��ȣ�������Һ��������д�����ɫ���������
����⣬����ʵ���в�������ɫ�����ΪCO2���塣
(1)�Խ��Ϳ����ڱ�����ˮ�м���ʯ��ʯ�Ʊ�HClO��ԭ��________________________________________________________________________
________________________________________________________________________��
(2)д��������е�һ�ݼ��ڶ�����Һ������Ӧ�����ӷ���ʽ��
��һ��________________________________________________________________________��
�ڶ���________________________________________________________________________��
(3)�Ը�����ѧ֪ʶ�Ʋ⣬�ڢڵ���Һ�к��е����ʣ������ܽ�ļ����������⣬�����е���������Ϊ(д��ѧʽ)__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��SO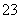 ��Fe2����Br����I����0.1 mol����Һ��ͨ���״���µ�Cl2��ͨ��Cl2���������Һ��������ӵ����ʵ����Ĺ�ϵͼ��ȷ����(����)
��Fe2����Br����I����0.1 mol����Һ��ͨ���״���µ�Cl2��ͨ��Cl2���������Һ��������ӵ����ʵ����Ĺ�ϵͼ��ȷ����(����)

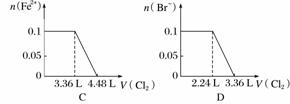
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����(COCl2)�����ϡ��Ƹ��ҩ�ȹ�ҵ����������;����ҵ�ϲ��ø�����CO��Cl2�ڻ���̿���ºϳɡ�
(1)ʵ�����г������Ʊ������Ļ�ѧ����ʽΪ________________________________________________________________________��
(3)ʵ�����п����ȷ�(CHCl3)��˫��ˮֱ�ӷ�Ӧ�Ʊ��������䷴Ӧ�Ļ�ѧ����ʽΪ________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ԭ�ӵ�һ�����Ӵӵڶ��ܼ�ԾǨ����һ�ܼ�ʱ������Ĺ��ӵIJ�����121.6 nm�����Ӵӵ����ܼ�ԾǨ���ڶ��ܼ�ʱ��������ӵIJ���Ϊ656.3 nm���Իش�
(1)���ֹ��ӵ�������˵�����ɡ�
(2)����ԭ���е��ӵ����ܼ��͵ڶ��ܼ���������ڶ��ܼ��͵�һ�ܼ����������˵��ԭ���е������Ƿ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ͼ(������Ϊ����������������Ϊ�������ʵ���)���Ӧ��ѡ������ϵ���(����)
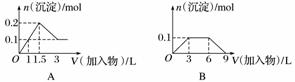
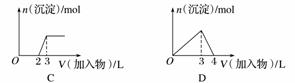
A����1 LŨ�Ⱦ�Ϊ0.1 mol·L��1��Ba(OH)2��NaAlO2�����Һ�м���0.1 mol·L��1��ϡH2SO4
B����1 LŨ�ȷֱ�Ϊ0.1 mol·L��1��0.3 mol·L��1��AlCl3��NH4Cl�Ļ����Һ�м���0.1 mol·L��1��ϡNaOH��Һ
C�����ռ���Һ�еμ�������Һ
D����AlCl3��Һ�еμӹ�����ˮ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com