
| A£® | 0.1 mol•L-1 NaHCO3ČÜŅŗÓė0.1 mol•L-1 NaOHČÜŅŗµČĢå»ż»ģŗĻ£¬ĖłµĆČÜŅŗÖŠ£ŗc£ØNa+£©£¾c£ØCO32-£©£¾c£ØHCO32-£©£¾c£ØOH-£© | |
| B£® | 20 mL 0.1 mol•L-1 CH3COONaČÜŅŗÓė10 mL 0.1 mol•L-1 HClČÜŅŗ»ģŗĻŗóČÜŅŗ³ŹĖįŠŌ£¬ĖłµĆČÜŅŗÖŠ£ŗc£ØCH3COO-£©£¾c£ØCl-£©£¾c£ØCH3COOH£©£¾c£ØH+£© | |
| C£® | ŹŅĪĀĻĀ£¬pH=2µÄŃĪĖįÓėpH=12µÄ°±Ė®µČĢå»ż»ģŗĻ£¬ĖłµĆČÜŅŗÖŠ£ŗc£ØCl-£©+c£ØH+£©£¾c£ØNH4+£©+c£ØOH-£© | |
| D£® | 0.1 mol•L-1 CH3COOHČÜŅŗÓė0.1 mol•L-1 NaOHČÜŅŗµČĢå»ż»ģŗĻ£¬ĖłµĆČÜŅŗÖŠ£ŗc£ØOH-£©£¾c£ØH+£©+c£ØCH3COOH£© |
·ÖĪö A.0.1 mol•L-1 NaHCO3ČÜŅŗÓė0.1 mol•L-1 NaOHČÜŅŗµČĢå»ż»ģŗĻ£¬¶žÕßĒ”ŗĆĶźČ«·“Ӧɜ³ÉNa2CO3£¬Ģ¼ĖįøłĄė×ÓĖ®½āµ¼ÖĀČÜŅŗ³Ź¼īŠŌ£¬µ«Ė®½ā³Ģ¶Č½ĻŠ”£¬Ė®µēĄėŅ²Éś³ÉĒāŃõøłĄė×Ó£»
B.20 mL 0.1 mol•L-1 CH3COONaČÜŅŗÓė10 mL 0.1 mol•L-1 HClČÜŅŗ»ģŗĻŗóČÜŅŗ³ŹĖįŠŌ£¬ČÜŅŗÖŠČÜÖŹĪŖµČĪļÖŹµÄĮæÅØ¶ČµÄCH3COONa”¢NaCl”¢CH3COOH£¬ČÜŅŗ³ŹĖįŠŌ£¬ĖµĆ÷“×ĖįµēĄė³Ģ¶Č“óÓŚ“×ĖįÄĘĖ®½ā³Ģ¶Č£¬µ«“×ĖįµēĄė³Ģ¶Č½ĻŠ”£»
C£®ŹŅĪĀĻĀ£¬pH=2µÄŃĪĖįÅØ¶ČŠ”ÓŚpH=12µÄ°±Ė®£¬¶žÕßµČĢå»ż»ģŗĻŗ󣬰±Ė®ÓŠŹ£Óą£¬ČÜŅŗ³Ź¼īŠŌ£¬µ«ČÜŅŗÖŠČŌČ»“ęŌŚµēŗÉŹŲŗć£¬øł¾ŻµēŗÉŹŲŗćÅŠ¶Ļ£»
D.0.1 mol•L-1 CH3COOHČÜŅŗÓė0.1 mol•L-1 NaOHČÜŅŗµČĢå»ż»ģŗĻ£¬¶žÕßĒ”ŗĆĶźČ«·“Ӧɜ³É“×ĖįÄĘ£¬ČÜŅŗÖŠ“ęŌŚÖŹ×ÓŹŲŗć£¬øł¾ŻÖŹ×ÓŹŲŗćÅŠ¶Ļ£®
½ā“š ½ā£ŗA.0.1 mol•L-1 NaHCO3ČÜŅŗÓė0.1 mol•L-1 NaOHČÜŅŗµČĢå»ż»ģŗĻ£¬¶žÕßĒ”ŗĆĶźČ«·“Ӧɜ³ÉNa2CO3£¬Ģ¼ĖįøłĄė×ÓĖ®½āµ¼ÖĀČÜŅŗ³Ź¼īŠŌ£¬µ«Ė®½ā³Ģ¶Č½ĻŠ”£¬Ė®µēĄėŅ²Éś³ÉĒāŃõøłĄė×Ó£¬ĖłŅŌĄė×ÓÅØ¶Č“óŠ”Ė³ŠņŹĒc£ØNa+£©£¾c£ØCO32-£©£¾c£ØOH-£©£¾c£ØHCO32-£©£¬¹ŹA“ķĪó£»
B.20 mL 0.1 mol•L-1 CH3COONaČÜŅŗÓė10 mL 0.1 mol•L-1 HClČÜŅŗ»ģŗĻŗóČÜŅŗ³ŹĖįŠŌ£¬ČÜŅŗÖŠČÜÖŹĪŖµČĪļÖŹµÄĮæÅØ¶ČµÄCH3COONa”¢NaCl”¢CH3COOH£¬ČÜŅŗ³ŹĖįŠŌ£¬ĖµĆ÷“×ĖįµēĄė³Ģ¶Č“óÓŚ“×ĖįÄĘĖ®½ā³Ģ¶Č£¬µ«“×ĖįµēĄė³Ģ¶Č½ĻŠ”£¬½įŗĻµēŗÉŹŲŗćµĆĄė×ÓÅØ¶Č“óŠ”Ė³ŠņŹĒ£ŗc£ØCH3COO-£©£¾c£ØCl-£©£¾c£ØCH3COOH£©£¾c£ØH+£©£¬¹ŹBÕżČ·£»
C£®ŹŅĪĀĻĀ£¬pH=2µÄŃĪĖįÅØ¶ČŠ”ÓŚpH=12µÄ°±Ė®£¬¶žÕßµČĢå»ż»ģŗĻŗ󣬰±Ė®ÓŠŹ£Óą£¬ČÜŅŗ³Ź¼īŠŌ£¬c£ØH+£©£¼c£ØOH-£©µ«ČÜŅŗÖŠČŌČ»“ęŌŚµēŗÉŹŲŗć£¬øł¾ŻµēŗÉŹŲŗćµĆc£ØCl-£©£¼c£ØNH4+£©£¬ĖłŅŌµĆc£ØCl-£©+c£ØH+£©£¼c£ØNH4+£©+c£ØOH-£©£¬¹ŹC“ķĪó£»
D.0.1 mol•L-1 CH3COOHČÜŅŗÓė0.1 mol•L-1 NaOHČÜŅŗµČĢå»ż»ģŗĻ£¬¶žÕßĒ”ŗĆĶźČ«·“Ӧɜ³É“×ĖįÄĘ£¬ČÜŅŗÖŠ“ęŌŚÖŹ×ÓŹŲŗć£¬øł¾ŻÖŹ×ÓŹŲŗćµĆc£ØOH-£©=c£ØH+£©+c£ØCH3COOH£©£¬¹ŹD“ķĪó£»
¹ŹŃ”B£®
µćĘĄ ±¾Ģāæ¼²éĄė×ÓÅØ¶Č“óŠ”±Č½Ļ£¬ĪŖøßĘµæ¼µć£¬²ąÖŲæ¼²éѧɜ·ÖĪöÅŠ¶ĻÄÜĮ¦£¬Ć÷Č·ČÜŅŗÖŠČÜÖŹ¼°ĘäŠŌÖŹŹĒ½ā±¾Ģā¹Ų¼ü£¬ČĪŗĪµē½āÖŹČÜŅŗÖŠ¶¼“ęŌŚµēŗÉŹŲŗć”¢ĪļĮĻŹŲŗćŗĶÖŹ×ÓŹŲŗć£¬ÕāŠ©ŹŲŗćÓėČÜŅŗĖį¼īŠŌ¼°ÅضČĪŽ¹Ų£¬ĢāÄæÄѶČÖŠµČ£®


 ŗ®¼ŁĢģµŲÖŲĒģ³ö°ęÉēĻµĮŠ“š°ø
ŗ®¼ŁĢģµŲÖŲĒģ³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
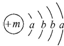 £¬XµÄŃōĄė×ÓÓėYµÄŅõĄė×ӵĵē×Ó²ć½į¹¹ĻąĶ¬£®ŌŖĖŲZ”¢W¾łĪŖ¶ĢÖÜĘŚŌŖĖŲ£¬ĖüĆĒŌ×ÓµÄ×īĶā²ćµē×ÓŹż¾łŹĒĘäµē×Ó²ćŹżµÄ2±¶£¬ZÓėYĻąĮŚĒŅZ”¢WÄÜŠĪ³ÉŅ»ÖÖWZ2ŠĶ·Ö×Ó£®
£¬XµÄŃōĄė×ÓÓėYµÄŅõĄė×ӵĵē×Ó²ć½į¹¹ĻąĶ¬£®ŌŖĖŲZ”¢W¾łĪŖ¶ĢÖÜĘŚŌŖĖŲ£¬ĖüĆĒŌ×ÓµÄ×īĶā²ćµē×ÓŹż¾łŹĒĘäµē×Ó²ćŹżµÄ2±¶£¬ZÓėYĻąĮŚĒŅZ”¢WÄÜŠĪ³ÉŅ»ÖÖWZ2ŠĶ·Ö×Ó£®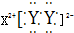

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŅŅĶéÓėCl2Č”“ś | B£® | ŅŅĻ©ÓėCl2¼Ó³É | ||
| C£® | ŅŅČ²ÓėHCl¼Ó³É | D£® | ŅŅ“¼ÓėÅØŃĪĖįČ”“ś |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
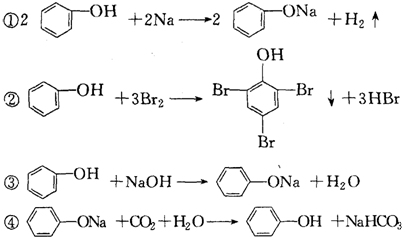
| A£® | ¢Ł¢Ū | B£® | ¢Ś¢Ü | C£® | ¢Ś | D£® | ¶¼²»æÉŅŌ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŌŚ³£ĪĀ³£Ń¹ĻĀĪŖŅŗĢå | B£® | ÄÜŹ¹ĖįŠŌKMnO4ČÜŅŗĶŹÉ« | ||
| C£® | æÉ×ŌÉķ¼Ó³ÉŠĪ³É¾ŪŅŅĻ© | D£® | ÄÜŹ¹äåµÄCCl4ČÜŅŗĶŹÉ« |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® |  | B£® |  | C£® | 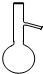 | D£® | 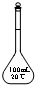 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Na2O±ČNa2O2ĪČ¶Ø | B£® | ¾ł²»æÉÓėĖ®·“Ó¦ | ||
| C£® | ¾łæÉÓėCO2·“Ó¦ | D£® | ¾łŹĒµäŠĶµÄ¼īŠŌŃõ»ÆĪļ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com