
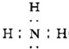
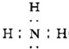

| 2b |
| a |
| 2b |
| a |
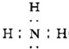 £¬HŗĶM·“Ӧɜ³ÉĢśŗĶŃõ»ÆĀĮ£¬ĒŅæɷųö“óĮæµÄČČ£¬“Ó¶ųÄÜŗø½ÓøÖ¹ģ£¬¹Ź“š°øĪŖ£ŗ
£¬HŗĶM·“Ӧɜ³ÉĢśŗĶŃõ»ÆĀĮ£¬ĒŅæɷųö“óĮæµÄČČ£¬“Ó¶ųÄÜŗø½ÓøÖ¹ģ£¬¹Ź“š°øĪŖ£ŗ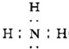 £»ŗø½ÓøÖ¹ģ£»
£»ŗø½ÓøÖ¹ģ£» £¬¹Ź“š°øĪŖ£ŗ
£¬¹Ź“š°øĪŖ£ŗ £»
£»| a |
| 2 |
| a |
| 2 |
| 2b |
| a |
| 2b |
| a |
| 2b |
| a |



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
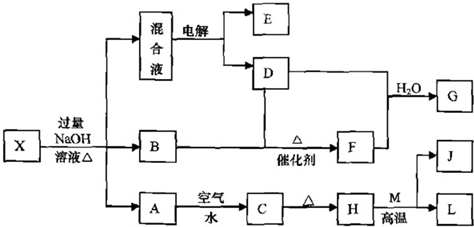
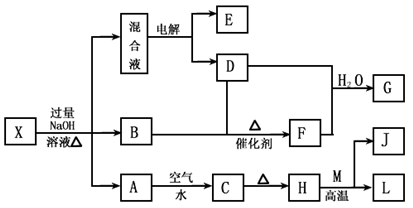
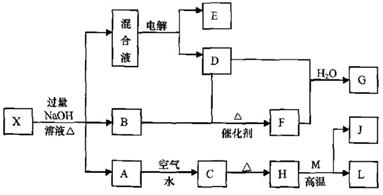
ÓŠŅ»»ÆŗĻĪļX£¬ĘäĖ®ČÜŅŗĪŖĒ³ĀĢÉ«£¬æÉ·¢ÉśČēĻĀµÄ×Ŗ»Æ¹ŲĻµ£Ø²æ·Ö·“Ó¦Īļ”¢Éś³ÉĪļŅŃĀŌ£©”£ĘäÖŠ£¬B”¢D”¢E”¢F¾łĪŖĪŽÉ«ĘųĢ壬M”¢LĪŖ³£¼ūµÄ½šŹōµ„ÖŹ£¬CĪŖÄŃČÜÓŚĖ®µÄŗģŗÖÉ«¹ĢĢ唣ŌŚ»ģŗĻŅŗÖŠ¼ÓČėBaCl2ČÜŅŗæÉÉś³É²»ČÜÓŚĻ”ŃĪĖįµÄ°×É«³Įµķ£¬HŗĶM·“Ó¦æɷųö“óĮæµÄČČ”£
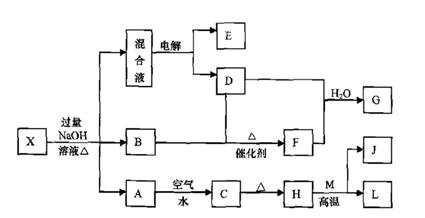
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©BµÄµē×ÓŹ½ĪŖ ”£
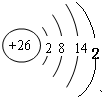
£Ø2£©»³öŌŖĖŲMµÄŌ×Ó½į¹¹Ź¾ŅāĶ¼ ”£
£Ø3£©ĪļÖŹXµÄ»ÆѧŹ½ĪŖ ”£
£Ø4£©µē½ā»ģŗĻŅŗŹ±Ńō¼«·“Ó¦Ź½ĪŖ ”£
£Ø5£©°“ŅŖĒ󊓳öÉĻŹö×Ŗ»Æ¹ŲĻµÖŠÓŠ¹Ų·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
¢Ł ŗ¬ÓŠLŌŖĖŲµÄ»ÆŗĻ·“Ó¦ ”£
¢Ś ŗ¬ÓŠLŌŖĖŲµÄÖĆ»»·“Ó¦ ”£
£Ø6£©ŅŃÖŖ![]()
![]() EĘųĢåŌŚDÖŠĶźČ«Č¼ÉÕÉś³ÉĪČ¶ØµÄ»ÆŗĻĪļŹ±£¬·Å³ö
EĘųĢåŌŚDÖŠĶźČ«Č¼ÉÕÉś³ÉĪČ¶ØµÄ»ÆŗĻĪļŹ±£¬·Å³ö![]() kJµÄČČĮ棬Š“³öEČ¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½ ”£
kJµÄČČĮ棬Š“³öEČ¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓŠŅ»»ÆŗĻĪļX£¬ĘäĖ®ČÜŅŗĪŖĒ³ĀĢÉ«£¬æÉ·¢ÉśČēĻĀµÄ×Ŗ»Æ¹ŲĻµ£Ø²æ·Ö·“Ó¦Īļ”¢Éś³ÉĪļŅŃĀŌ£©”£ĘäÖŠ£¬B”¢D”¢E”¢F¾łĪŖĪŽÉ«ĘųĢ壬M”¢LĪŖ³£¼ūµÄ½šŹōµ„ÖŹ£¬CĪŖÄŃČÜÓŚĖ®µÄŗģŗÖÉ«¹ĢĢ唣ŌŚ»ģŗĻŅŗÖŠ¼ÓČėBaCl2ČÜŅŗæÉÉś³É²»ČÜÓŚĻ”ŃĪĖįµÄ°×É«³Įµķ£¬HŗĶM·“Ó¦æɷųö“óĮæµÄČČ”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©BµÄµē×ÓŹ½ĪŖ ”£
£Ø2£©»³öŌŖĖŲMµÄŌ×Ó½į¹¹Ź¾ŅāĶ¼ ”£
£Ø3£©ĪļÖŹXµÄ»ÆѧŹ½ĪŖ ”£
£Ø4£©µē½ā»ģŗĻŅŗŹ±Ńō¼«·“Ó¦Ź½ĪŖ ”£
£Ø5£©°“ŅŖĒ󊓳öÉĻŹö×Ŗ»Æ¹ŲĻµÖŠÓŠ¹Ų·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
¢Ł ŗ¬ÓŠLŌŖĖŲµÄ»ÆŗĻ·“Ó¦ ”£
¢Ś ŗ¬ÓŠLŌŖĖŲµÄÖĆ»»·“Ó¦ ”£
£Ø6£©ŅŃÖŖ![]()
 EĘųĢåŌŚDÖŠĶźČ«Č¼ÉÕÉś³ÉĪČ¶ØµÄ»ÆŗĻĪļŹ±£¬·Å³ö
EĘųĢåŌŚDÖŠĶźČ«Č¼ÉÕÉś³ÉĪČ¶ØµÄ»ÆŗĻĪļŹ±£¬·Å³ö![]() kJµÄČČĮ棬Š“³öEČ¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½ ”£
kJµÄČČĮ棬Š“³öEČ¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010-2011ѧğŗÓ±±Ź”øßČżĻĀѧʌµŚŅ»“Īµ÷ŃŠæ¼ŹŌ£ØĄķ×Ū£©»Æѧ²æ·Ö ĢāŠĶ£ŗĢīæÕĢā
ÓŠŅ»»ÆŗĻĪļX£¬ĘäĖ®ČÜŅŗĪŖĒ³ĀĢÉ«£¬æÉ·¢ÉśČēĻĀµÄ×Ŗ»Æ¹ŲĻµ£Ø²æ·Ö·“Ó¦Īļ”¢Éś³ÉĪļŅŃĀŌ£©”£ĘäÖŠ£¬B”¢D”¢E”¢F¾łĪŖĪŽÉ«ĘųĢ壬M”¢LĪŖ³£¼ūµÄ½šŹōµ„ÖŹ£¬CĪŖÄŃČÜÓŚĖ®µÄŗģŗÖÉ«¹ĢĢ唣ŌŚ»ģŗĻŅŗÖŠ¼ÓČėBaCl2ČÜŅŗæÉÉś³É²»ČÜÓŚĻ”ŃĪĖįµÄ°×É«³Įµķ£¬HŗĶM·“Ó¦æɷųö“óĮæµÄČČ”£
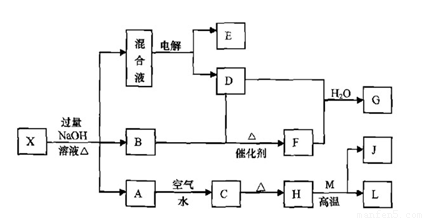
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©BµÄµē×ÓŹ½ĪŖ ”£
£Ø2£©»³öŌŖĖŲMµÄŌ×Ó½į¹¹Ź¾ŅāĶ¼ ”£
£Ø3£©ĪļÖŹXµÄ»ÆѧŹ½ĪŖ ”£
£Ø4£©µē½ā»ģŗĻŅŗŹ±Ńō¼«·“Ó¦Ź½ĪŖ ”£
£Ø5£©°“ŅŖĒ󊓳öÉĻŹö×Ŗ»Æ¹ŲĻµÖŠÓŠ¹Ų·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
¢Ł ŗ¬ÓŠLŌŖĖŲµÄ»ÆŗĻ·“Ó¦ ”£
¢Ś ŗ¬ÓŠLŌŖĖŲµÄÖĆ»»·“Ó¦ ”£
£Ø6£©ŅŃÖŖ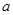
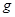 EĘųĢåŌŚDÖŠĶźČ«Č¼ÉÕÉś³ÉĪČ¶ØµÄ»ÆŗĻĪļŹ±£¬·Å³ö
EĘųĢåŌŚDÖŠĶźČ«Č¼ÉÕÉś³ÉĪČ¶ØµÄ»ÆŗĻĪļŹ±£¬·Å³ö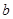 kJµÄČČĮ棬Š“³öEČ¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½
ӣ
kJµÄČČĮ棬Š“³öEČ¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½
ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗÕć½Ź”Ä£ÄāĢā ĢāŠĶ£ŗĶʶĻĢā
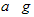 EĘųĢåŌŚDÖŠĶźČ«Č¼ÉÕÉś³ÉĪČ¶ØµÄ»ÆŗĻĪļŹ±£¬·Å³öb kJµÄČČĮ棬Š“³öøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½________________________ ”£
EĘųĢåŌŚDÖŠĶźČ«Č¼ÉÕÉś³ÉĪČ¶ØµÄ»ÆŗĻĪļŹ±£¬·Å³öb kJµÄČČĮ棬Š“³öøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½________________________ ”£ ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓŠŅ»»ÆŗĻĪļX£¬ĘäĖ®ČÜŅŗĪŖĒ³ĀĢÉ«£¬æÉ·¢ÉśČēĻĀµÄ×Ŗ»Æ¹ŲĻµ£Ø²æ·Ö·“Ó¦Īļ”¢Éś³ÉĪļŅŃĀŌ£©”£ĘäÖŠ£¬B”¢D”¢E”¢F¾łĪŖĪŽÉ«ĘųĢ壬M”¢LĪŖ³£¼ūµÄ½šŹōµ„ÖŹ£¬CĪŖÄŃČÜÓŚĖ®µÄŗģŗÖÉ«¹ĢĢ唣ŌŚ»ģŗĻŅŗÖŠ¼ÓČėBaCl2ČÜŅŗæÉÉś³É²»ČÜÓŚĻ”ŃĪĖįµÄ°×É«³Įµķ£¬HŗĶM·“Ó¦æɷųö“óĮæµÄČČ”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
 |
£Ø1£©BµÄµē×ÓŹ½ĪŖ ”£
£Ø2£©»³öŌŖĖŲMµÄŌ×Ó½į¹¹Ź¾ŅāĶ¼ ”£
£Ø3£©ĪļÖŹXµÄ»ÆѧŹ½ĪŖ ”£
£Ø4£©µē½ā»ģŗĻŅŗŹ±Ńō¼«·“Ó¦Ź½ĪŖ ”£
£Ø5£©°“ŅŖĒ󊓳öÉĻŹö×Ŗ»Æ¹ŲĻµÖŠÓŠ¹Ų·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
¢Ł ŗ¬ÓŠLŌŖĖŲµÄ»ÆŗĻ·“Ó¦ ”£
¢Ś ŗ¬ÓŠLŌŖĖŲµÄÖĆ»»·“Ó¦ ”£
£Ø6£©ŅŃÖŖ![]()
![]() EĘųĢåŌŚDÖŠĶźČ«Č¼ÉÕÉś³ÉĪČ¶ØµÄ»ÆŗĻĪļŹ±£¬·Å³ö
EĘųĢåŌŚDÖŠĶźČ«Č¼ÉÕÉś³ÉĪČ¶ØµÄ»ÆŗĻĪļŹ±£¬·Å³ö![]() kJµÄČČĮ棬Š“³öEČ¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½ ”£
kJµÄČČĮ棬Š“³öEČ¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com