
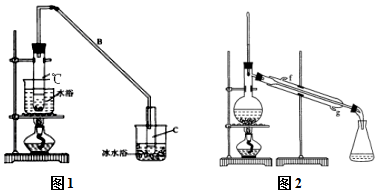
 ij��ѧС���������������������װ�ã���ͼ1�����Ի������Ʊ�����ϩ
ij��ѧС���������������������װ�ã���ͼ1�����Ի������Ʊ�����ϩ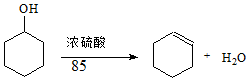
| �ܶȣ�g/cm3�� | �۵㣨�棩 | �е㣨�棩 | �ܽ��� | |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | -103 | 83 | ������ˮ |
���� ��1���ٸ���ʵ��������ϩ��֪������װ��A�����Ƭ�������Ƿ�ֹ���У�����B�Ƚϳ���������������ĽӴ�������������ǵ����ͽ����ɵ�������������������
�ڱ�ˮԡ��Ŀ���ǽ��ͻ���ϩ�������¶ȣ�ʹ��Һ����
��2���ٻ���ϩ�������Ȼ�����Һ�����ܶȱ�ˮС���ֲ��ϩ���ϲ㣬���ڷ�Һ��ϩ��Ʒ�л�������������ͻ�����������Na2CO3��Һϴ�ӻ��������ƿɳ�ȥ�
��Ϊ��ʹ������Ч�����ã���ȴˮ�������ܵ��¿ڽ��룬�Ͽڳ�����ʯ������ˮ��Ӧ�����������ƣ�
�۸��ݱ������ݿ�֪����ֻ���ϩ�ķе�Ϊ83�棻
��3�����ݻ����û�й̶��ķе㣬���������й̶��ķе㣬�ݴ˿��жϲ�Ʒ�Ĵ��ȣ�
��� �⣺��1���ٸ�������ϩʵ���֪ʶ������װ��A�����Ƭ�������Ƿ�ֹ���У��������ɵĻ���ϩ�ķе�Ϊ83�棬Ҫ�õ�Һ̬����ϩ������B���˵���������������ã����ڻ���ϩ������
�ʴ�Ϊ����ֹ���У�������
�ڱ�ˮԡ��Ŀ���ǽ��ͻ���ϩ�������¶ȣ�ʹ��Һ������ֹ����ϩ�ӷ���
�ʴ�Ϊ����ֹ����ϩ�ӷ�����������ϩ��
��2���ٻ���ϩ�����࣬�������Ȼ�����Һ�����ܶȱ�ˮС�������á��ֲ��ϩ���ϲ㣻����ϩ�к���̼̼˫�������Ա�KMnO4��Һ������ϡH2SO4���ܳ�ȥ�������������µ��������ʣ�����Na2CO3��Һϴ�ӻ��������ƣ������뻷��ϩ��Ӧ���������������ʷ�Ӧ��CD��ѡ��
�ʴ�Ϊ���ϣ�CD��
��Ϊ��ʹ������Ч�����ã���ȴˮ�������ܵ��¿ڼ�g�ڽ��룻��ʯ������ˮ��Ӧ�����������ƣ���ȥ�˲�����ˮ���õ������Ļ���ϩ��
�ʴ�Ϊ��g����ˮ��
�۸��ݱ������ݿ�֪����ֻ���ϩ�ķе�Ϊ83�棬���ռ���ƷӦ�����¶���83�����ң�
A������ʱ��70�濪ʼ�ռ���Ʒ����ǰ�ռ�����Ʒ�л������ʣ�ʵ�ʲ����������۲�������A����
B��������ʵ���������ˣ���ȡ�Ļ���ϩ�����ʵ�������ʵ���ƵõĻ���ϩ��Ʒ�����������۲�������B����
C�����ֲ�Ʒ�л��л����������²ⶨ���ĵĻ������������ƵõĻ���ϩ��Ʒ�����������۲�������C��ȷ��
�ʴ�Ϊ��83�棻C��
��3�������Ʒ�뾫Ʒ�ɼ�������ƣ��۲��Ƿ�������������������壬���Ǿ�Ʒ��������ݻ����û�й̶��ķе㣬���������й̶��ķе㣬ͨ���ⶨ����ϩ��Ʒ�ͻ���ϩ��Ʒ�ķе㣬Ҳ���жϲ�Ʒ�Ĵ��ȣ�
�ʴ�Ϊ��BC��
���� �����ۺϿ����˻���ϩ���Ʊ���������ѧ������֪ʶ����������Ŀ�Ѷ��еȣ�ע�����ʵ��ԭ���ͷ������ر���ʵ��Ļ���������ѧϰ��ע����ۣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.1mol/LFeC13��Һ�У�A13+��NH4+��Cl-��SCN- | |
| B�� | ʹ���ȱ��ɫ����Һ�У�Mg2+��K+��SO42-��NO3- | |
| C�� | $\frac{{K}_{W}}{c��{H}^{+}��}$=1��10-15mol/L����Һ�У�Na+��Fe3+��I-��AlO2- | |
| D�� | ˮ�����c��H+��=1��10-13mol/L����Һ�У�K+��Na+��CO32-��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | K +����MnO 4 -��Cl -��SO 4 2- | B�� | Na +��CO 3 2-��Cl -��SO 4 2- | ||
| C�� | NO 3 -��Na +��HCO 3 -��Ba 2+ | D�� | Na +��NO 3 -��NH 4 +��SO 4 2- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
 Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ���
Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��Ӧʱ��/min | 0 | 10 | 20 | 30 | 40 | 50 |
| c��N2O��/mol•L-1 | 0.100 | 0.090 | 0.080 | 0.070 | 0.060 | 0.050 |
| ��Ӧʱ��/min | 60 | 70 | 80 | 90 | 100 | |
| c��N2O��/mol•L-1 | 0.040 | 0.030 | 0.020 | 0.010 | 0.000 |
| A�� | 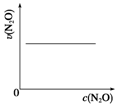 | B�� |  | C�� |  | D�� |  |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | Ԫ�صķǽ����Դ���Ϊc��b��a | |
| B�� | a-�Ļ�ԭ������c-�Ļ�ԭ�� | |
| C�� | d������3��Ԫ�ؾ����γ����ӻ����� | |
| D�� | Ԫ��a��b��c������ߺ���ͻ��ϼ۵Ĵ����ͷֱ�Ϊ0��4��6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com