
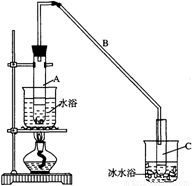
ij��ѧС���������������������װ�ã���ͼ�����Ի������Ʊ�����ϩ��
��֪��
|
|
�ܶ� ��g/cm3�� |
�۵� ���棩 |
�е� ���棩 |
�ܽ��� |
|
������ |
0.96 |
25 |
161 |
������ˮ |
|
����ϩ |
0.81 |
��103 |
83 |
������ˮ |
��1���Ʊ���Ʒ
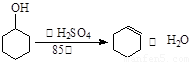
��12.5mL�����������Թ�A�У��ټ���1mLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ�������� ������B���˵�������е������� ��
���Թ�C���ڱ�ˮԡ�е�Ŀ���� ��
��2���Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ�� �㣨��ϡ����¡�������Һ���� �������ţ�ϴ�ӡ�
A��KMnO4��Һ B��ϡH2SO4 C��Na2CO3��Һ
���ٽ�����ϩ��ͼװ��������ȴˮ�� �ڽ��롣����ʱҪ������ʯ�ң�Ŀ����: ��
���ռ���Ʒʱ�����Ƶ��¶�Ӧ�� ���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ���� �ߣߣߣߣߣߣ�
A������ʱ��70�濪ʼ�ռ���Ʒ B��������ʵ����������
C���Ʊ���Ʒʱ���������Ʒһ������
��3���������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ������������ǣߣߣߣߣߣߣ�
A�������Ը��������Һ B���ý����� C���ⶨ�е�
��4���ɻ���ϩ��ת��Ϊ�������Ļ�ѧ����ʽΪ���ߣߣߣߣߣߣߣߣߣߣߣߣߣ�
(1) �� ��ֹ���У� ������ �ڷ�ֹ����ϩ�Ļӷ���
(2) ���ϣ� C�� ��g����ȥˮ�֡� �� 83�� �� C��
��3��B��C��
��4������ϩ + H2O����������
��������
�����������1���ټ���Һ��ʱ��Ϊ�˷�ֹҺ�屬�У������ü����ʯ�����Ƭ���ӷ������ʷ�ӦʱΪ����������ʣ�һ��Ҫ����������װ�ã�����B�������������ã��ڷ�Ӧ���ɵIJ���е�ϸߣ��ӷ���Ӧ���ñ�ˮ��ȴ����ֹ����ϩ�Ļӷ�����2���������ܶȱ�ˮС����ˮ���ʱ������ϩ���ϲ㣻Ҫ��ȥ��Ʒ�е������ʣ�����̼���Ƶȼ�����Һ��ȥ����ʵ����һ�����������ȴ������ʱ������ʯ��Ŀ������ˮ��Ӧ����ֹ���Ʒһ������������ռ���Ʒʱ�����Ƶ��¶�Ӧ�û���ϩ�ӷ���������2��������������Һ�У������¶ȿɿ�����83�桪��161�棬����Ϊ��ˮԡ���Ⱥ����������ĵȷ���ɿ����¶ȵ�һЩ 83�漴�ɣ�ʵ���ƵõĻ���ϩ��Ʒ�����������۲�����˵������ϩ������ʧ�ˣ��ʴ�ΪC����3����Ʒ�к��л����������Ʒ�Ӧ���һ����е㲻�̶����뻷��ϩ�е㲻ͬ��
���㣺���������Ʊ�ʵ���в�����Ŀ�ġ�ԭ�������������й����⡣


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


| �ܶȣ�g/cm3�� | �۵㣨�棩 | �е㣨�棩 | �ܽ��� | ������ | 0.96 | 25 | 161 | ������ˮ | ����ϩ | 0.81 | -103 | 83 | ������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


| �ܶȣ�g/cm3�� | �۵㣨�棩 | �е㣨�棩 | �ܽ��� | ������ | 0.96 | 25 | 161 | ������ˮ | ����ϩ | 0.81 | -103 | 83 | ������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧС���������������������װ�ã���ͼ��)�Ի������Ʊ�����ϩ��

��֪��
| �ܶȣ�g��cm-3) | �۵㣨��)�� | �㣨��) | �ܽ��� | |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | -103 | 83 | ������ˮ |

ͼ��
(1)�Ʊ���Ʒ
��12.5 mL�����������Թ�A�У��ټ���1 mLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ��������_____________������B���˵�������е�������_______________��
���Թ�C���ڱ�ˮԡ�е�Ŀ����_________________________________________________��
��2���Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ��кͱ���ʳ��ˮ�������á��ֲ㣬����ϩ��_______________�㣨��ϡ����¡�������Һ����_______________�������ţ�ϴ�ӡ�
a.KMnO4��Һ
b.ϡH2SO4
c.Na2CO3��Һ

ͼ��
���ٽ�����ϩ��ͼ��װ��������ȴˮ��____________�ڽ��롣����ʱҪ������ʯ�ң�Ŀ����____________��
���ռ���Ʒʱ�����Ƶ��¶�Ӧ��____________���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ����________________________��
a.����ʱ��70 �濪ʼ�ռ���Ʒ
b.������ʵ����������
c.�Ʊ���Ʒʱ���������Ʒһ������
��3���������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������������____________��
a.�ø������������Һ b.�ý����� c.�ⶨ�е�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ӱ�ʡ��ɽһ�и߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
(11��)ij��ѧС���������������������װ��(��ͼ)���Ի������Ʊ�����ϩ��
��֪��

| | �ܶ�(g/cm3) | �۵�(��) | �е�(��) | �ܽ��� |
| ���Ѵ� | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | ��103 | 83 | ������ˮ |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��㶫ʡ÷������������ѧ�߶�5���¿���ѧ�Ծ����������� ���ͣ�ʵ����
��20�֣�ij��ѧС���������������������װ�ã���ͼ�����Ի������Ʊ�����ϩ
��1���Ʊ���Ʒ
��12.5mL�����������Թ�A�У��ټ���lmLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ��������____________������B���˵�������е�������____ ��
���Թ�C���ڱ�ˮԡ�е�Ŀ����_____________________________��
��2)�Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��______��(���ϻ���)����Һ����_________ (������)ϴ�ӡ�
A��KMnO4��Һ B��ϡH2SO4 C��Na2CO3��Һ
���ٽ�����ϩ����ͼװ��������ȴˮ��_________�ڽ��롣����ʱҪ������ʯ�ң�Ŀ����______________ ____��
���ռ���Ʒʱ�����Ƶ��¶�Ӧ��_________���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ����____________________
A������ʱ��70�濪ʼ�ռ���Ʒ
B��������ʵ����������
C���Ʊ���Ʒʱ���������Ʒһ������
��3���������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������������_________��
A�������Ը��������Һ B���ý����� C���ⶨ�е�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com