

【题目】已知某“84消毒液”瓶体部分标签如图所示,该“84消毒液”通常稀释100倍(体积之比)后使用。请回答下列问题:
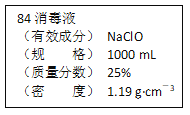
(1)该“84消毒液”的物质的量浓度约为_____mol·L-1。
(2)取用任意体积的该盐溶液时,下列物理量中会随所取体积的多少而变化的是________(填字母)。
A.溶液中NaClO的物质的量 B.溶液的浓度
C.溶液中NaClO的摩尔质量 D.溶液的密度
(3)该同学参阅该“84消毒液”的配方,欲用NaClO固体配制480 mL含NaClO质量分数为25%的消毒液。回答下列问题。
①如图所示的仪器中,有些是不需要,配制上述溶液还需要玻璃仪器_______

②需要称量NaClO固体的质量为_______ g
(4)“84消毒液”与稀硫酸混合使用可增强消毒能力,某消毒小组人员用98%(密度为1.84 g·cm-3)的浓硫酸配制200 mL 2.3 mol·L-1的稀硫酸用于增强“84消毒液”的消毒能力。
①所配制的稀硫酸中,H+的物质的量浓度为________mol·L-1。
②需用浓硫酸的体积为________ mL。
③若所配制的稀硫酸浓度偏小,则下列可能的原因分析中正确的是_______。
A.配制前,容量瓶中有少量蒸馏水 B.量取浓硫酸时,仰视液体的凹液面
C.未冷却,立即转移至容量瓶定容 D.定容时,仰视溶液的凹液面
【答案】4.0 A 玻璃棒,胶头滴管 148.8 4.6 25.0 D
【解析】
(1)该“84消毒液”的物质的量浓度约为![]() mol·L-1。
mol·L-1。
(2)A.溶液中NaClO的物质的量和溶液体积有关,溶液体积越大,NaClO的物质的量越多;
B.溶液是均一稳定的,其浓度和体积无关。
C.NaClO的摩尔质量是74.5g/mol,和溶液体积无关。
D.溶液是均一稳定的,溶液的密度和体积无关。
故选A。
(3)配制480 mL含NaClO质量分数为25%的消毒液,由于实验室没有480mL的容量瓶,所以要用500mL的容量瓶配制500mL的溶液。需要先计算出需要NaClO的质量,然后用天平称出NaClO固体,放在烧杯中,加入蒸馏水,用玻璃棒搅拌溶解后转移到容量瓶中,用蒸馏水洗涤所用的烧杯和玻璃棒2~3次,把洗涤液也转移到容量瓶中,然后直接向容量瓶中加蒸馏水到离刻度线1~2cm的地方,改用胶头滴管滴加蒸馏水到刻度线,最后上下颠倒摇匀。
①需要的仪器有烧杯、玻璃棒、托盘天平、500mL容量瓶、胶头滴管,除了给出的烧杯、托盘天平和容量瓶外,还需要玻璃棒和胶头滴管。
②计算称量NaClO固体的质量:500mL×1.19g·cm-3×25%=148.8g。
(4)①2.3 mol·L-1的稀硫酸中,H+的物质的量浓度为2.3 mol·L-1×2=4.6mol·L-1。
②浓硫酸的物质的量浓度为![]() mol/L,根据稀释前后溶质的物质的量不变,可计算配制200 mL 2.3 mol·L-1的稀硫酸需要的浓硫酸的体积为
mol/L,根据稀释前后溶质的物质的量不变,可计算配制200 mL 2.3 mol·L-1的稀硫酸需要的浓硫酸的体积为![]() ,即25.0 mL。
,即25.0 mL。
③A.配制前,容量瓶中有少量蒸馏水,不影响溶质的物质的量,也不影响溶液的体积,所以对所配溶液的浓度无影响;
B.量取浓硫酸时,仰视液体的凹液面,会导致所取的浓硫酸偏多,配制的稀硫酸的浓度偏高;
C.未冷却,立即转移至容量瓶定容会导致溶液体积偏小,配制的稀硫酸的浓度偏高;
D.定容时,仰视溶液的凹液面会导致溶液体积偏大,配制的稀硫酸的浓度偏低。
故选D。


科目:高中化学 来源: 题型:
【题目】用NA表示阿伏加德罗常数的值。下列叙述正确的是( )
A. 常温常压下,11.2L二氧化硫所含的氧原子数等于NA
B. 0.5molH2O所含的电子数为9NA
C. 8.0gCu2S和CuO的混合物中含有铜原子数为0.1NA
D. 300mL2mol·L-1蔗糖溶液中所含分子数为0.6NA
查看答案和解析>>
科目:高中化学 来源: 题型:
【题目】(1)质量比为16∶7∶6的三种气体SO2、CO、NO,分子个数之比为_____________;氧原子个数之比为____________。
(2)在标准状况下,6.8g PH3与标准状况下_______L CH4含有相同数目的H原子。
(3)某气体氧化物化学式为RO2,在标准状况下,1.28 g该氧化物的体积为448 mL,则该氧化物的摩尔质量为________,R的相对原子质量为________。
(4)273 K、1.01×105 Pa时气态单质X2的密度为1.25 g·L-1,则X的相对原子质量为________
(5)相同温度和压强条件下,一定体积的气态氢化物H2R的质量是等体积NH3的2倍,则R的相对原子质量为____________。
查看答案和解析>>
科目:高中化学 来源: 题型:
【题目】粗铜精炼后的阳极泥中含有Cu、Au(金)和PbSO4等杂质,湿法处理阳极泥进行综合利用的工艺流程如图所示:
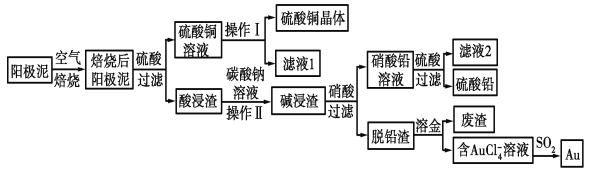
(1)电解精炼含铜、金、铅的粗铜时,电解液应该用________溶液作电解液,电解时阳极的电极反应式为___________________________和Cu-2e-===Cu2+。
(2)完成操作Ⅰ的主要步骤有:__________________,过滤,洗涤,干燥。
(3)写出用SO2还原AuCl4-的离子反应方程式____________________________。
(4)为了减少废液排放、充分利用有用资源,工业上将滤液1并入硫酸铜溶液进行循环操作,请指出流程图中另一处类似的做法________________________。
(5)用离子方程式表示加入碳酸钠溶液的作用:___________________________。[已知298 K时,Ksp(PbCO3)=1.46×10-13,Ksp(PbSO4)=1.82×10-8]。当溶液中c(SO42-)=0.2mol/L时,c(CO32-)=_______________mol/L。(结果保留2位有效数字)
查看答案和解析>>
科目:高中化学 来源: 题型:
【题目】著名的“侯氏制碱法”主要反应原理是:NH3+CO2+H2O+NaCl=NaHCO3↓+NH4Cl。若实验室根据此原理制备少量的Na2CO3,主要实验包括:制取NH3和CO2→生成NaHCO3→分离NaHCO3→制取Na2CO3 四个步骤。下列实验选用的主要仪器或主要步骤不正确的是
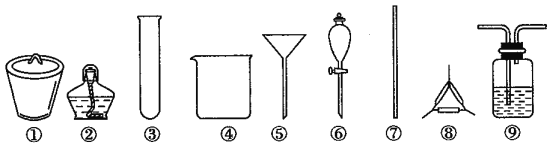
A. 制取氨气,可选用②③
B. 分离 NaHCO3,可选用④⑤⑦
C. 制取 Na2CO3,可选用①②⑦⑧
D. 制取 NaHCO3时,应先在⑨中通入CO2后再加入氨水
查看答案和解析>>
科目:高中化学 来源: 题型:
【题目】向V mL 0.1 mol/L氨水中滴加等物质的量浓度的稀H2SO4,测得混合溶液的温度和pOH [pOH=-lgc(OH-)]随着加入稀硫酸的体积的变化如图所示(实线为温度变化,虚线为pOH变化),下列说法不正确的是

A. V =40
B. b点时溶液的pOH > pH
C. a、b、c三点由水电离的c(OH-)依次减小
D. a、b、d三点对应NH3·H2O的电离常数:K(b)>K(d)>K(a)
查看答案和解析>>
科目:高中化学 来源: 题型:
【题目】硫酸四氨合铜晶体([Cu(NH3)4]SO4·H2O)常用作杀虫剂,媒染剂,在碱性镀铜中也常用作电镀液的主要成分,在工业上用途广泛。常温下该物质在空气中不稳定,受热时易发生分解。某化学兴趣小组以Cu粉、3mol/L的硫酸、浓氨水、10% NaOH溶液、95%的乙醇溶液、0.500 mol/L稀盐酸、0.500 mol/L的NaOH溶液来合成硫酸四氨合铜晶体并测定其纯度。
I.CuSO4溶液的制备
①称取4g铜粉,在A仪器中灼烧10分钟并不断搅拌,放置冷却。
②在蒸发皿中加入30mL 3mol/L的硫酸,将A中固体慢慢放入其中,加热并不断搅拌。
③趁热过滤得蓝色溶液。
(1)A仪器的名称为________________________________。
(2)某同学在实验中有1.5g的铜粉剩余,该同学将制得的CuSO4溶液倒入另一蒸发皿中加热浓缩至有晶膜出现,冷却析出的晶体中含有白色粉末,试解释其原因__________________________________________。
II.晶体的制备
将上述制备的CuSO4溶液按如图所示进行操作
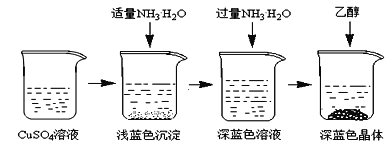
(3)已知浅蓝色沉淀的成分为Cu2(OH)2SO4,试写出生成此沉淀的离子反应方程式___________________。
(4)析出晶体时采用加入乙醇的方法,而不是浓缩结晶的原因是________________________。
III.氨含量的测定
精确称取wg晶体,加适量水溶解,注入如图所示的三颈瓶中,然后逐滴加入足量10%NaOH溶液,通入水蒸气,将样品液中的氨全部蒸出,并用蒸馏水冲洗导管内壁,用V1mL0.5mol/L的盐酸标准溶液完全吸收。取下接收瓶,用0.5mol/L NaOH标准溶液滴定过剩的HCl(选用甲基橙作指示剂),到终点时消耗V2mLNaOH溶液。
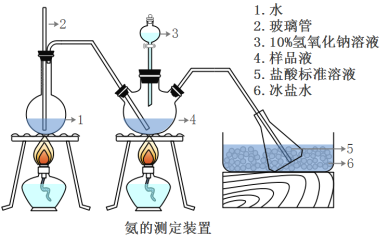
(5)A装置中长玻璃管的作用_________________,样品中氨的质量分数的表达式_______。
(6)下列实验操作可能使氨含量测定结果偏高的原因是____________________。
A.滴定时未用NaOH标准溶液润洗滴定管。
B.读数时,滴定前平视,滴定后俯视。
C.滴定过程中选用酚酞作指示剂。
D.取下接收瓶前,未用蒸馏水冲洗插入接收瓶中的导管外壁。
查看答案和解析>>
科目:高中化学 来源: 题型:
【题目】氮和碳的化合物在生产生活中应用广泛。
(1)①氯胺(NH2Cl)的电子式为_______________。
②NH2Cl与水反应生成强氧化性的物质,可作长效缓释消毒剂,该反应的化学方程式为__________________。
(2)用焦炭还原NO的反应为:2NO(g)+C(s)![]() N2(g)+CO2(g),向容积均为1L的甲、乙、丙三个恒容恒温(反应温度分别为400℃、400℃、T℃)容器中分别加入足量的焦炭和一定量的NO,测得各容器中n(NO)随反应时间t的变化情况如下表所示:
N2(g)+CO2(g),向容积均为1L的甲、乙、丙三个恒容恒温(反应温度分别为400℃、400℃、T℃)容器中分别加入足量的焦炭和一定量的NO,测得各容器中n(NO)随反应时间t的变化情况如下表所示:
t/min | 0 | 40 | 80 | 120 | 160 |
n(NO)(甲容器)/mol | 2.00 | 1.50 | 1.10 | 0.80 | 0.80 |
n(NO)(乙容器)/mol | 1.00 | 0.80 | 0.65 | 0.53 | 0.45 |
n(NO)(丙容器)/mol | 2.00 | 1.45 | 1.00 | 1.00 | 1.00 |
①该反应为_______________(填“放热”或“吸热”)反应。
②乙容器在200min达到平衡状态,则0~200min内用NO的浓度变化表示的平均反应速率v(NO)=___________。
(3)用焦炭还原NO2的反应为:2NO2(g)+2C(s)![]() N2(g)+2CO2(g),在恒温条件下,1mol NO2和足量C发生该反应,测得平衡时NO2和CO2的物质的量浓度与平衡总压的关系如下图所示:
N2(g)+2CO2(g),在恒温条件下,1mol NO2和足量C发生该反应,测得平衡时NO2和CO2的物质的量浓度与平衡总压的关系如下图所示:

①A、B两点的浓度平衡常数关系:Kc(A)_____Kc(B)(填“<”或“>”或“=”)。
②A、B、C三点中NO2的转化率最高的是__________(填“A”或“B”或“C”)点。
③计算C点时该反应的压强平衡常数Kp(C)=________(Kp是用平衡分压代替平衡浓度计算,分压=总压×物质的量分数)。
(4)最新研究发现,用隔膜电解法可以处理高浓度乙醛废水。原理:使用惰性电极电解,乙醛分别在阴、阳极转化为乙醇和乙酸,总反应为:2CH3CHO+H2O![]() CH3CH2OH+CH3COOH。实验室中,以一定浓度的乙醛-Na2SO4溶液为电解质溶液,模拟乙醛废水的处理过程,其装置示意图如下图所示:
CH3CH2OH+CH3COOH。实验室中,以一定浓度的乙醛-Na2SO4溶液为电解质溶液,模拟乙醛废水的处理过程,其装置示意图如下图所示:

①试写出电解过程中,阴极的电极反应式:_________________________。
②在实际处理过程中,当电路中I=50A时,10min处理乙醛8.8g,则电流效率为__(计算结果保留3位有效数字,每个电子的电量为1.6×10-19C,电流效率=实际反应所需电量/电路中通过电量×100%)。
查看答案和解析>>
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com