
������ʵ�����(������)����ȷ����(����)
A����Ũ��ˮ�ӵ�ʢ��AgCl������Թ��У�AgCl������ȫ�ܽ⣬��������Һ��c(Ag��)��c(Cl��)>Ksp(AgCl)
B�����ʵ���֮��Ϊ1:2��Na2O2��NaHCO3�Ĺ���������ܱ������г�ּ��ȷ�Ӧ�����Ĺ�����Na2CO3
C����FeBr2��Һ��ͨ������������������Ӧ�����Һ�еμ�KSCN��Һ�������Һ��Ϊ��ɫ����ô��FeI2��Һ��ͨ��������������Ҳ�����ͬ��������
D����ij�������ͨ��Ʒ����Һ�У���Һ����ɫ������������Cl2
���������⿼��������ܽ�ƽ�⡢������ԭ��Ӧ������ļ���ȣ����ڿ��鿼���Ի�ѧ��Ӧԭ�������⡣������֪AgCl������ȫ�ܽ⣬��c(Ag��)��c(Cl��)������Ksp(AgCl)��A��������ʵ���֮��Ϊ1:2��Na2O2��NaHCO3�Ĺ����������ʱ������Ӧ��2Na2O2��4NaHCO3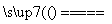 4Na2CO3��O2����2H2O���������ΪNa2CO3��B����ȷ����ԭ�ԣ�I��>Fe2��>Br������FeI2��Һ��ͨ��������������I���ȱ��������μ�KSCN��Һ�������ɫ��C���������������к���SO2��Cl2����n(SO2):n(Cl2)��1:1��ͨ��Ʒ����Һ�У���Һ����ɫ��D�����
4Na2CO3��O2����2H2O���������ΪNa2CO3��B����ȷ����ԭ�ԣ�I��>Fe2��>Br������FeI2��Һ��ͨ��������������I���ȱ��������μ�KSCN��Һ�������ɫ��C���������������к���SO2��Cl2����n(SO2):n(Cl2)��1:1��ͨ��Ʒ����Һ�У���Һ����ɫ��D�����
�𰸣�B



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й���ͬϵ���˵���У�����ȷ���� (����)
A��ͬϵ�������ͬ�����ʽ
B��ͬϵ���ܷ���ͬһͨʽ
C��ͬϵ���У����ڵ�ͬϵ��˴�����������һ��CH2ԭ����
D��ͬϵ��Ļ�ѧ���ʻ������ƣ�����������̼ԭ�������Ӷ��ʹ����Ա仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵��������� (����)
A����ҵ�ϵ������״̬��Al2O3�Ʊ�Al�漰������ԭ��Ӧ (2012���㶫���ۣ�10B)
B���Ĵ������ؽ�ʹ���˴����ֲģ��ֲ��ǺϽ� (2009���㶫��5��)
C���������϶��ǵ��壬�ǽ������϶��Ǿ�Ե�� (2008��ɽ�����ۣ�9A)
D���Ͻ�����п��ܺ��зǽ���Ԫ�� (2009��ɽ�����ۣ�9A)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͭ�����ã����������һ����ɫ���壬Ϊ�˽�ͭ�ڿ����еĸ�ʴ�����ij��ѧ��ȤС���ռ�����ͭ���������ɫ�������̽��������������Ϻ������ɫ���ʿ�����ͭ��̼�� �Ρ�
�Ρ�
��С��ͬѧ������ͼװ�ý���ʵ��(���ּг�������)��
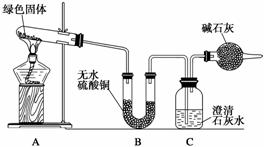
�ٶ��Թ��ڵ���ɫ������м��ȣ�����ȫ�ֽ⣬�۲쵽Aװ������ɫ������ɺ�ɫ��Bװ������ˮ����ͭ�����ɫ��Cװ���г���ʯ��ˮ����ǡ�
��ȡ�������Ⱥ����ɵĺ�ɫ�������Թ��У�����ϡ���ᣬ�۲쵽��ɫ�������ܽ⣬��Һ�����ɫ��
��ȡ����������ɫ��Һ���Թ��У�����һ���ྻ����˿��
�۲쵽��˿�����к�ɫ����������
��ش��������⣺
(1)��ɫ�����к��е�Ԫ����______________________________________________��
(2)���Ⱥ��Թ���ʣ��ĺ�ɫ������________________________________________��
(3)�������ɫ������һ�ִ�������仯ѧʽ������________________________�����ȷֽ�Ļ�ѧ����ʽΪ________________________________________________________
__________________________________________ ______________________________��
______________________________��
(4)����ʵ�鲽����еķ�Ӧ�����ӷ���ʽΪ__________________________________
________________________________________________________________________��
(5)ʵ��װ�����ĸ���ܵ�������_________________________________________��
(6)�����B��C��װ�öԵ����ܷ�ﵽʵ��Ŀ��________(��ܡ����ܡ�)��Ϊʲô��________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£�ˮ�ĵ���ﵽƽ�⣺H2OH����OH��������������ȷ����(����)
A����ˮ���ȣ�ƽ��������Ӧ�����ƶ���KW����
B����ˮ�м����������ᣬƽ�����淴Ӧ�����ƶ���c(H��)����
C����ˮ�м�������NaOH���壬ƽ�����淴Ӧ�����ƶ���c(OH��)����
D����ˮ�м�������CH3COONa���壬ƽ��������Ӧ�����ƶ���c(OH��)��c(H��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������ֿ�ʼ��Ű�ҹ��ֵ���������SO2����ɿ�����Ⱦ����Ҫԭ�������Ƽ�ѭ�����ɳ�ȥSO2��
(1)�Ƽ�ѭ�����У�����ҺΪNa2SO3��Һ�������շ�Ӧ�����ӷ���ʽ��________________��
(2)��֪H2SO3�ĵ��볣��ΪK1��1.54��10��2��K2��1.02��10��7��H2CO3�ĵ��볣��ΪK1��4.30��10��7��K2��5.60��10��11�������������Թ������ ________________��
A��CO ��HSO
��HSO B��HCO
B��HCO ��HSO
��HSO
C��SO ��HCO
��HCO D��H2SO3��HCO
D��H2SO3��HCO
(3)����Һ����SO2�Ĺ����У�pH��n(SO ):n(HSO
):n(HSO )�仯��ϵ���±���
)�仯��ϵ���±���
| n(SO | 91:9 | 1:1 | 9:91 |
| pH | 8.2 | 7.2 | 6.2 |
���ϱ��ж�NaHSO3��Һ��________________�ԣ���ԭ���ĽǶȽ���ԭ��________________��
����NaHSO3��Һ����Ũ�ȹ�ϵ����ȷ����________(ѡ����ĸ)��
A��c(Na��)��2c(SO )��c(HSO
)��c(HSO )
)
B��c(Na��)>c(HSO )>c(H��)>c(SO
)>c(H��)>c(SO )>c(OH��)
)>c(OH��)
C��c(H2SO3)��c(H��)��c(SO )��c(OH��)
)��c(OH��)
D��c(Na��)��c(H��)��2c(SO )��c(HSO
)��c(HSO )��c(OH��)
)��c(OH��)
(4)������Һ��pH����ԼΪ6ʱ����������������������ʾ��ͼ���£�
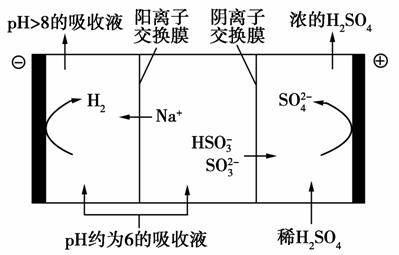
������Һ���������е��ܷ�Ӧ����ʽ��________________��
�ڵ��缫����1 mol����ת��ʱ��������Ϊ________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������е�A��B��C��D��E����Ԫ�أ�ԭ��������������A��D��C��E�ֱ�ͬ���壬AΪ�ǽ���Ԫ�أ���A��B��ԭ������֮�͵���C��ԭ��������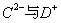 �ĺ����������ȡ�������˵����ȷ����
�ĺ����������ȡ�������˵����ȷ����
A��B��Aֻ�����BA3������
B��C��D��E�γɵĻ�����ˮ��Һ�����Լ���
C��A��B��C�γɵĻ�����һ�����ܷ���ˮ�ⷴӦ
D��E���������Ӧ��ˮ����ֻ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ba(OH)2��Һ����������Һ�У�ʹSO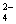 ȫ��ת����BaSO4��������ʱ��Ԫ��
ȫ��ת����BaSO4��������ʱ��Ԫ��
����Ҫ������ʽ��
 A��Al3+ B��Al(OH)3 C��AlO2�� D��Al3+ ��Al(OH)3
A��Al3+ B��Al(OH)3 C��AlO2�� D��Al3+ ��Al(OH)3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͭ��һ����Ũ���ᷴӦ���õ�����ͭ��Һ��NO2��N2O4��NO�Ļ�����壬��Щ������1.68 L O2(��״��)��Ϻ�ͨ��ˮ�У�����������ȫ��ˮ�����������ᡣ������������ͭ��Һ�м���5 mol��L-1 NaOH��Һ��Cu2��ǡ����ȫ������������NaOH��Һ�����
A��60 mL B��45 mL C��30 mL D��15 mL
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com