
Na��Cu��O��Si��S��Cl�dz���������Ԫ�ء�
(1)Naλ��Ԫ�����ڱ���________���ڵ�________�壻S�Ļ�̬ԭ�Ӻ�����________��δ�ɶԵ��ӣ�Si�Ļ�̬ԭ�Ӻ�������Ų�ʽΪ________________________��
(2)�á�>����<����գ�
| ��һ������ | ���Ӱ뾶 | �۵� | ���� |
| Si____S | O2��____Na�� | NaCl____Si | H2SO4____HClO4 |
(3)CuCl(s)��O2��Ӧ����CuCl2(s)��һ�ֺ�ɫ���塣��25 �桢101 kPa�£���֪�÷�Ӧÿ����1 mol CuCl(s)������44.4 kJ���÷�Ӧ���Ȼ�ѧ����ʽ��________________________________________________________________________
________________________________________________________________________��
(4)ClO2������ˮ�ľ�������ҵ�Ͽ���Cl2����NaClO2��Һ��ȡClO2��д���÷�Ӧ�����ӷ���ʽ�����������ת�Ƶķ������Ŀ��________________________________________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ֲ��ӷ��͵ijɷ�֮һ�����Ľṹ��ʽΪ��HO�� ��CH2CH=CH2�����������в���ȷ����( )
��CH2CH=CH2�����������в���ȷ����( )
A��1mol������������4molH2������Ӧ
B��1mol������������4mol�巢����Ӧ
C�������ӿ����ȩ������Ӧ�����ɾۺ���
D����������ˮ�е��ܽ��С�ڱ�����ˮ�е��ܽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����þ��ҽҩ����������ҵӦ�ù㷺������ þ��ԭ�Ƚ��Ʊ��ߴ�����þ��һ���µ�̽��������þ��(��Ҫ�ɷ�ΪMgCO3��������FeCO3)Ϊԭ���Ʊ��ߴ�����þ��ʵ���������£�
þ��ԭ�Ƚ��Ʊ��ߴ�����þ��һ���µ�̽��������þ��(��Ҫ�ɷ�ΪMgCO3��������FeCO3)Ϊԭ���Ʊ��ߴ�����þ��ʵ���������£�
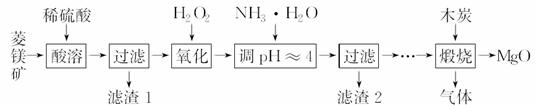
(1)MgCO3��ϡ���ᷴӦ�����ӷ���ʽΪ__________________________________��
(2)����H2O2����ʱ��������Ӧ�Ļ�ѧ����ʽΪ____________________________��
(3)����2�ijɷ���________(�ѧʽ)��
(4)���չ��̴������·�Ӧ��
2MgSO4��C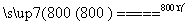 2MgO��2SO2����CO2��
2MgO��2SO2����CO2��
MgSO4��C MgO��SO2����CO��
MgO��SO2����CO��
MgSO4��3C MgO��S����3CO��
MgO��S����3CO��
������ͼװ�ö����ղ�����������зֲ����ջ��ռ���

��D���ռ������������________(�ѧʽ)��
��B��ʢ�ŵ���Һ������________(����ĸ)��
a��NaOH��Һ b��Na2CO3��Һ
c��ϡ���� d��KMnO4��Һ
��A�еõ��ĵ���ɫ���������ȵ�NaOH��Һ��Ӧ��������Ԫ�����̬Ϊ��4��д���÷�Ӧ�����ӷ���ʽ��__________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����X���߶�BaSO4�������ϲ�����ԣ�ҽѧ���ڽ�������ϵͳ��X������ʱ������BaSO4���ڷ���Ӱ�������ּ���ֶγ�Ϊ�����ӡ�
(1)ҽѧ�Ͻ��б�����ʱΪʲô����BaCO3��(�����ӷ���ʽ��ʾ)________________________________________________________________________��
(2)ij����С��Ϊ��̽��BaSO4���ܽ�ȣ��ֱ�����BaSO4���룺
a��5 mL ˮ��
b��40 mL 0.2 mol/L ��Ba(OH)2��Һ��
c��20 mL 0.5 mol/L��Na2SO4 ��Һ��
d��40 mL 0.1 mol/L ��H2SO4 ��Һ�У��ܽ������͡�
�����ϸ���Һ�У�Ba2����Ũ���ɴ�С��˳��Ϊ________��
A��b��a��c��d B��b��a��d��c
C��a��d��c��b D��a��b��d��c
����֪298 Kʱ��Ksp(BaSO4)��1.1��10��10�����������£���Һb�е�SO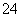 Ũ��Ϊ________ mol/L����Һc��Ba2����Ũ��Ϊ________ mol/L��
Ũ��Ϊ________ mol/L����Һc��Ba2����Ũ��Ϊ________ mol/L��
��ijͬѧȡͬ���������Һb����Һdֱ�ӻ�ϣ�����Һ��pHΪ________(������Һ�����Ϊ���ǰ����Һ�����֮��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ���ɵ���̼ԭ�ӹ��ɵ�ƽ��ṹ����̼���ϣ�ʯīϩ�в���̼ԭ�ӱ���������ƽ��ṹ�ᷢ���ı䣬ת��Ϊ����ʯīϩ[��ͼ(b)��ʾ]��
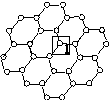 ��
��
(a)ʯīϩ�ṹ����������(b)����ʯīϩ�ṹ
(1)ͼ(a)�У�1��C������C�γɦҼ��ĸ���Ϊ________��
(2)ͼ(b)�У�1��C���ӻ���ʽ��________����C������C�γɵļ���________(�>����<������)ͼ(a)��1��C������C�γɵļ��ǡ�
(3)����ͼ(b)��ʾ������ʯīϩ��ɢ��H2O�У�������ʯīϩ�п���H2O�γ������ԭ����________(��Ԫ�ط���)��
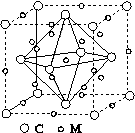
(4)ʯīϩ��ת��Ϊ����ϩ(C60)��ij����M��C60���Ʊ�һ�ֵ��³������ϣ�������ͼ��ʾ��Mԭ��λ�ھ������������ڲ����þ�����Mԭ�ӵĸ���Ϊ________���ò��ϵĻ�ѧʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y��Z��RΪǰ������Ԫ�أ���ԭ��������������XY2�Ǻ���ɫ���壻X����Ԫ�ؿ��γ�XH3��Z��̬ԭ�ӵ�M����K���������ȣ�R2����3d�������9�����ӡ�
��ش��������⣺
(1)Y��̬ԭ�ӵĵ����Ų�ʽ��________��Z���������е�һ��������������Ԫ����________��
(2)XY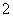 �����幹����________��R2����ˮ�������У��ṩ�µ��ӶԵ�ԭ����________��
�����幹����________��R2����ˮ�������У��ṩ�µ��ӶԵ�ԭ����________��
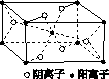
(3)Z��ijԪ���γɵĻ�����ľ�����ͼ��ʾ���������������������ӵĸ�������________��
(4)��R���ʵķ�ĩ����XH3��Ũ��Һ�У�ͨ��Y2����ַ�Ӧ����Һ������ɫ���÷�Ӧ�����ӷ���ʽ��____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y��Z��W��R���ַ����ڲ�ͬ����Ķ�����Ԫ�أ�ԭ��������������X����̬�⻯�������ֻ��һ�Թµ��Ӷԣ�Y��Z��W������������Ӧ��ˮ��������������Ӧ��
(1)X�����ڱ��е�λ����________��Z3���ĺ�������Ų�ʽΪ________��
(2)Y��Z��R�ĵ�һ�����ܵĴ�С˳��Ϊ________(��Ԫ�ط��ű�ʾ)��
(3)W���������������ķ��ӹ���Ϊ________����������������ˮ����������Һ���������е�W�ӻ��������Ϊ________��
(4)��R�ĵ�����Y������������Ӧ��ˮ�����ϣ��䷴Ӧ�����ӷ���ʽΪ________________________________________________________________________��
(5)ͼK332��ʾΪY��Z�γɵĺϽ��ṹ���������1 mol Y�ĸúϽ�����������ˮ�г�ַ�Ӧ���ų���״������������Ϊ________L��

ͼK332
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ���¶��£����淴ӦA(g)��3B(g) 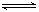 2C(g)�ﵽƽ��״̬�ı�־��
2C(g)�ﵽƽ��״̬�ı�־��
A. C���ɵ�������A�ֽ������2�����
B. ��λʱ������n mol A��ͬʱ����3n mol B
C. A��B��C��Ũ�Ȳ��ٱ仯
D. A��B��C�ķ�������Ϊ1��3��2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ȼ�ϵ����KOH��ҺΪ�������Һ�������йظõ�ص���������ȷ����
(����)
A��������ӦʽΪ��O2��2H2O��4e��===4OH��
B������һ��ʱ����Һ��KOH�����ʵ���Ũ�Ȳ���
C����ȼ�ϵ�ص��ܷ�Ӧ����ʽΪ��2H2��O2===2H2O
D���øõ�ص��CuCl2��Һ������2.24 L Cl2(��״��)ʱ����0.4 mol ����ת��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com