
| A£®ŹÆÄ«Č¼ÉÕÉś³ÉCOĘųĢåµÄČČ»Æѧ·½³ĢŹ½ĪŖ£ŗ ””””2C(ŹÆÄ«)+O2(g)=2CO(g)”÷H=-110.5kJ/mol |
| B£®C(ŹÆÄ«)²»ĶźČ«Č¼ÉÕ£¬Éś³ÉCO2ŗĶCOĮ½ÖÖĘųĢåŹ±£¬æÉ·ÅČČ283.0kJ |
| C£®C(ŹÆÄ«)ŗĶCO2(g)·“Ӧɜ³ÉCO(g)µÄ·“Ó¦ŹĒĪüČČ·“Ó¦ |
| D£®Čō½šøÕŹÆµÄČ¼ÉÕÉś³ÉCO2ĘųĢå·Å³öµÄČČĮæ“óÓŚŹÆÄ«£¬ŌņŹÆÄ«±ä³É½šøÕŹÆµÄ±ä»ÆŹĒ·ÅČČ·“Ó¦ |



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¢Ł¢Ś¢Ū | B£®¢Ś¢Ū | C£®¢Ł¢Ś | D£®¢Ł¢Ū¢Ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā


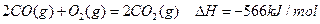
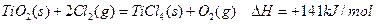
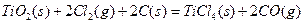 µÄ
µÄ =________________”£
=________________”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ŹĒĪüČČ·“Ó¦ |
| B£®ŹĒ·ÅČČ·“Ó¦ |
| C£®ŹĒģŲ¼õÉŁµÄ·“Ó¦ |
| D£®ģŲŌö“óŠ§Ó¦“óÓŚÄÜĮ抧Ӧ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
 aŹ±£¬lmol H2ĶźČ«Č¼ÉÕÉś³ÉŅŗĢ¬Ė®£¬·Å³ö2
aŹ±£¬lmol H2ĶźČ«Č¼ÉÕÉś³ÉŅŗĢ¬Ė®£¬·Å³ö2 85£®8kJµÄČČĮ棻1 mol CH4ĶźČ«Č¼ÉÕÉś³ÉŅŗĢ¬Ė®ŗĶCO2ĘųĢ壬·Å³ö890£®3kJµÄČČĮ攣ĻĀĮŠČČ»Æѧ·½³ĢŹ½ŹéŠ“ÕżČ·µÄŹĒ £Ø £©
85£®8kJµÄČČĮ棻1 mol CH4ĶźČ«Č¼ÉÕÉś³ÉŅŗĢ¬Ė®ŗĶCO2ĘųĢ壬·Å³ö890£®3kJµÄČČĮ攣ĻĀĮŠČČ»Æѧ·½³ĢŹ½ŹéŠ“ÕżČ·µÄŹĒ £Ø £©| A£®2H2£Øg£©+ O2£Øg£©=2H2O£Ø1£©£»”÷H=-285£®8kJ£Æmol |
B£®CH4£Øg £©+2 O2£Øg£©=CO2£Øg£©+2H2O£Ø1£©£»”÷H=-890£®3kJ£Æmol £©+2 O2£Øg£©=CO2£Øg£©+2H2O£Ø1£©£»”÷H=-890£®3kJ£Æmol |
| C£®CH4£Øg£©+2 O2£Øg£©=CO2£Øg£©+2H2O£Øg£©£»”÷H=-890£®3kJ£Æmol |
| D£®CH4£Øg£©+2O2£Øg£©=CO2£Øg£©+2H2O£Ø1£©£»”÷H=+890£®3kJ£Æmol |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
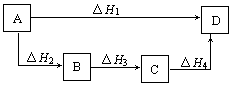
A£®”÷H1=”÷H2=”÷H3=”÷H 4 4 | B£®”÷H1+”÷H2=”÷H3+”÷H4 |
| C£®”÷H1+”÷H2+”÷H3=”÷H4 | D£®”÷H1=”÷H2+”÷H3+”÷H4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗ¼ĘĖćĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com