
 TiOCl2+2HCl��������650��850���£�������ͨ���������Ѻ�̿�۵Ļ����ɵõ����Ȼ��Ѻ�һ���ж����塣��ͼ��ʵ�����Ʊ�TiCl4�IJ���װ�á�
TiOCl2+2HCl��������650��850���£�������ͨ���������Ѻ�̿�۵Ļ����ɵõ����Ȼ��Ѻ�һ���ж����塣��ͼ��ʵ�����Ʊ�TiCl4�IJ���װ�á�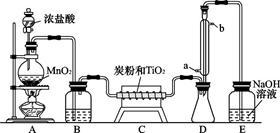
| ��� | װ��ȱ�ݺͲ���֮�� |
| �� | |
| �� | |
| �� | |
 TiCl4+2CO
TiCl4+2CO

 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
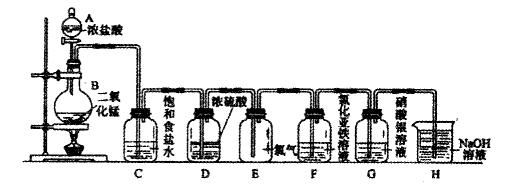
 ��2��C��ʢ�б���ʳ��ˮ���������� ��D��Ũ����������� ��
��2��C��ʢ�б���ʳ��ˮ���������� ��D��Ũ����������� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
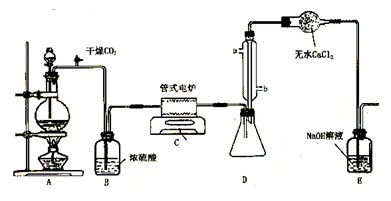
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
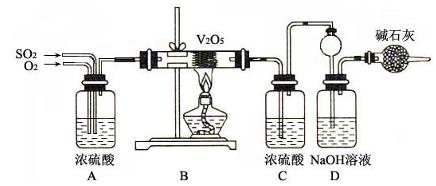
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ������ͼ��ʾ(װ�ÿ��ظ�ʹ��)���ش��������⣺
������ͼ��ʾ(װ�ÿ��ظ�ʹ��)���ش��������⣺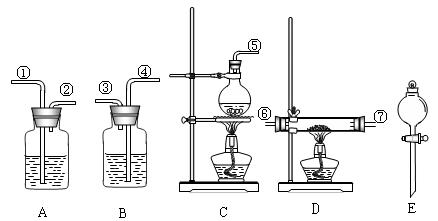
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
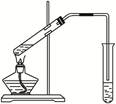
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ֻ�Тڢ� | B��ֻ�Тۢ� | C��ֻ�Т٢� | D��ֻ�Т٢� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���Խ�̿��ˮ��ȡˮú������������ |
| B��������п�Ȼ��ý�����ϡ���ᷴӦ��ȡ���� |
| C���ɻ������糧�ṩ�������ˮ�������� |
| D�����ø�Ч������̫����ʹ��ˮ�ֽ�������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com