

��1���������a��c���ر�b������е������ǣ���Ҫʱ�����Ȣ�д���ò�ʵ��������漰��Ӧ�Ļ�ѧ����ʽ��______________��________________��___________________��
��2�����������е�ϡHNO3����Ũ���ᣬ����a��c���ر�b����һ��۲죬���Կ�������û��ˮ�еĵ��ܿ�������ð��������������ʺ���ɫ��Һ��������Ϊ��ɫ���Խ�����һ����____________________________��
��3����ʵ�飨1���У�Ҫʹ���������ʼ�ձ�����ɫ��Ӧ������������____________________
��4����ʵ��װ�õ���Ҫ�ŵ���________________________����Ҫȱ����__________________��
��1��û��ˮ�еĵ��ܿ�������ð����ƿ����������ɫ��ɺ���ɫ���ٱ����ɫ?
3Cu+8HNO3��ϡ���T�T3Cu(NO3)2+2NO��+4H2O?
2NO+O2�T�T2NO2?
3NO2+H2O�T�T2HNO3+NO
��2�����������ɫ��NO2��H2O��Ӧ������ɫ��NO
��3���ȹر�a������b��c����һ������ٹر�b������a?
��4����Ӧ����ʱ������ֹͣ��ȱ��β������װ�ã��������Ⱦ?
��������1������a��c���ر�b����ʱ������Ӧ3Cu+8HNO3�T�T3Cu(NO3)2+2NO��+4H2O�ݳ�NO������У�����ӦΪ�����ܿ�������ð�����ݳ���������ɫ�����ɫ�����NO2�ֱ�ˮ���ձ�Ϊ��ɫ��?
��2����ΪŨHNO3��ӦΪCu+4HNO3��Ũ���T�TCu(NO3)2+2NO2��+2H2O�ݳ�NO2Ϊ����ɫ��������з�����Ӧ3NO2+H2O�T�T2HNO3+NO��?
��3������������ʼ����ɫ�����������O2�����£������ͨ��CO2��ʹCO2������Ȼ����ͨ��NO����ʱNO����CO2���������Ӵ��������������ɫ��?
��4���ŵ�����ʱ������Ӧ��ֹͣ����ȱ��β������װ�ã��ݳ�������������Ⱦ������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
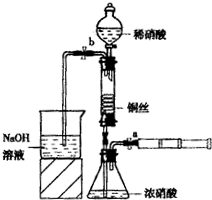 ��2011?��������ģ��ijͬѧ��������װ��ʵ��ͭ��Ũ��ϡ���ᷴӦ���������£�
��2011?��������ģ��ijͬѧ��������װ��ʵ��ͭ��Ũ��ϡ���ᷴӦ���������£�| ʵ���� | ˮ��/0C | Һ�������߶� | ||
| 1 | 25 | �����Թ�
| ||
| 2 | 50 | �����Թ�
| ||
| 3 | 0 | Һ����������ʵ��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijͬѧ������װ��ʵ��ͭ��Ũ��ϡ���ᷴӦ���������£�
ijͬѧ������װ��ʵ��ͭ��Ũ��ϡ���ᷴӦ���������£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣�ijͬѧ������װ��ʵ��ͭ��Ũ��ϡ���ᷴӦ���������£�
I��ȡһ��ͭ˿����ϡ�����ȥͭ��[��Ҫ�ɷ���Cu2��OH��2CO3]��
��ϴ�Ӻ��ͭ˿���������϶������״��
����ͼ��ʾ������������������ԡ�װ�뻯ѧ�Լ���
��1������I������Ӧ�����ӷ���ʽ��______________________��
��2�����̢�ͭ˿��������״��Ŀ����______________________��
��3�����̢�ĺ����������£�
��ΪʹŨ������ͭ˿�Ӵ���������______________________��������ɫ����϶�ʱ�����ע����ʹ��Ӧֹͣ���ر�a��ȡ��ע������
�ڻ�����һע������b�ͷ�Һ©�������������ܳ���ϡ����ʱ���ر�b��a���ɼ���ɫ���������ϡ������������ܵ�ʵ��Ŀ����______________________��
��4����֪��NO+NO2+2NaOH 2NaNO2+H2O��2NO2+2NaOH NaNO3+NaNO2+H2O��NO��NO2�Ļ���������ɿɱ�ʾΪNOx�����û������ͨ��NaOH��Һ����ȫ����ʱ��x��ȡֵ��ΧӦΪ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ������ѧ�ڵ�����ģ�⿼�Ի�ѧ�Ծ� ���ͣ�ʵ����
��10�֣�ijͬѧ������װ��ʵ��ͭ��Ũ��ϡ���ᷴӦ���������£�
I��ȡһ��ͭ˿����ϡ�����ȥͭ��[��Ҫ�ɷ���Cu2��OH��2CO3]��
��ϴ�Ӻ��ͭ˿���������϶������״��
����ͼ��ʾ������������������ԡ�װ�뻯ѧ�Լ���

��1������I������Ӧ�����ӷ���ʽ��______________________��
��2�����̢�ͭ˿��������״��Ŀ����______________________��
��3�����̢�ĺ����������£�
��ΪʹŨ������ͭ˿�Ӵ���������______________________��������ɫ����϶�ʱ�����ע����ʹ��Ӧֹͣ���ر�a��ȡ��ע������
�ڻ�����һע������b�ͷ�Һ©�������������ܳ���ϡ����ʱ���ر�b��a���ɼ���ɫ���������ϡ������������ܵ�ʵ��Ŀ����______________________��
��4����֪��NO+NO2+2NaOH
2NaNO2+H2O��2NO2+2NaOH
NaNO3+NaNO2+H2O��NO��NO2�Ļ���������ɿɱ�ʾΪNOx�����û������ͨ��NaOH��Һ����ȫ����ʱ��x��ȡֵ��ΧӦΪ______________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com