
ĻÖÓŠĻĀĮŠĪļÖŹ£ŗ¢ŁNa2CO3 ¢ŚĶ ¢ŪĀČ»ÆĒā ¢ÜCO2 ¢ŻNaHSO4 ¢ŽBa(OH)2 ¢ßĒāŃõ»ÆĢś½ŗĢå ¢ą°±Ė® ¢įĻ”ĻõĖį ¢āKI
£Ø1£©°“ĪļÖŹµÄ·ÖĄą·½·ØĢīŠ“±ķøńµÄæհד¦(ĢīĪļÖŹ±ąŗÅ)
| ·ÖĄą±ź×¼ | µē½āÖŹ | ŃĪ | ·Ēµē½āÖŹ | »ģŗĻĪļ |
| ŹōÓŚøĆĄą µÄĪļÖŹ | | | | |
£Ø1£©¢Ł¢Ū¢Ż¢Ž¢ā ¢Ł¢Ż¢ā ¢Ü ¢ß¢ą¢į (øĆŠ”Ģāø÷æÕĀ©Ń””¢“ķŃ”¾ł²»µĆ·Ö)
£Ø2£©Ba(OH)2+2HCl = BaCl2+2H2O»ņ Ba(OH)2+2HNO3 = Ba(NO3)2+2H2O
£Ø3£©O2 + 4I£ + 2 H2O = 2I2 + 4OH£
½āĪöŹŌĢā·ÖĪö£ŗ£Ø2£©·ūŗĻĄė×Ó·“Ó¦H£«£«OH£= H2OÓ¦ĪŖĒæĖįŗĶĒæ¼ī·“Ó¦£¬ĒŅ²»ÄÜÉś³É³Įµķ”¢ĘųĢåµČ²śĪļ£¬ĖłŅŌÓ¦ÓĆĀČ»ÆĒā”¢Ļ”ĻõĖįÓŚBa(OH)2·“Ó¦£»£Ø3£©µāĄė×Ó¾ßÓŠ½ĻĒæµÄ»¹ŌŠŌ£¬øł¾ŻĢāŅāĘä±»Ńõ»ÆĪŖµāµ„ÖŹ£¬ČÜŅŗĪŖÖŠŠŌ»·¾³£¬·½³ĢŹ½ĪŖO2 + 4I£ + 2 H2O = 2I2 + 4OH£”£
æ¼µć£ŗæ¼²éĪļÖŹ·ÖĄąŗĶĄė×Ó·½³ĢŹ½ŹéŠ“µČÓŠ¹ŲĪŹĢā”£


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ
| A£®”°¹ā»ÆѧŃĢĪķ”±”°ĻõĖįŠĶĖįÓź”±µÄŠĪ³É¶¼ÓėµŖŃõ»ÆŗĻĪļÓŠ¹Ų |
| B£®øł¾Ż·ÖÉ¢ÖŹĪ¢Į£Ö±¾¶“óŠ”æÉŅŌ½«·ÖÉ¢Ļµ·ÖĪŖČÜŅŗ”¢½ŗĢåŗĶ×ĒŅŗ |
| C£®SiO2æÉÓĆÓŚÖĘŌģ¹āµ¼ĻĖĪ¬£¬ĘäŠŌÖŹĪČ¶Ø£¬²»ČÜÓŚĒæĖį”¢Ēæ¼ī |
| D£®Ń껚µÄĪå²ŹēĶ·×ŹĒijŠ©½šŹōŌŖĖŲµÄŠŌÖŹµÄÕ¹ĻÖ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĢśŹĒČĖĄą½ĻŌēŹ¹ÓĆµÄ½šŹōÖ®Ņ»”£ŌĖÓĆĖłŃ§ÖŖŹ¶£¬»Ų“šĻĀĮŠĪŹĢā”£
£Ø1£©¼ų±šFe(OH)3½ŗĢåŗĶFeCl3ČÜŅŗµÄ·½·ØŹĒ ”£
£Ø2£©µē×Ó¹¤ŅµÓĆFeCl3ČÜŅŗøÆŹ“·óŌŚ¾ųŌµ°åÉĻµÄĶ£¬ÖĘŌģÓ”Ė¢µēĀ·°å£¬ĒėŠ“³öFeCl3ČÜŅŗÓėĶ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ ”£
£Ø3£©Ä³ŃŠ¾æŠŌѧĻ°Š”×éĪŖ²ā¶ØFeCl3øÆŹ“ĶŗóĖłµĆČÜŅŗµÄ×é³É£¬½ųŠŠĮĖČēĻĀŹµŃé£ŗ
¢ŁČ”ÉŁĮæ“ż²āČÜŅŗ£¬µĪČėKSCNČÜŅŗ³ŹŗģÉ«£¬Ōņ“ż²āŅŗÖŠŗ¬ÓŠµÄ½šŹōŃōĄė×ÓŹĒ £»¢ŚČÜŅŗ×é³ÉµÄ²ā¶Ø£ŗČ”50.0mL“ż²āČÜŅŗ£¬¼ÓČė×ćĮæµÄAgNO3ČÜŅŗ£¬µĆ21.525g°×É«³Įµķ”£ŌņČÜŅŗÖŠc(Cl£)£½ mol”¤L£1”£
¢ŪŃéÖ¤øĆČÜŅŗÖŠŗ¬ÓŠFe2+£¬ÕżČ·µÄŹµŃé·½·ØŹĒ ”£
A£®¹Ū²ģČÜŅŗŹĒ·ń³ŹĒ³ĀĢÉ«
B£®Č”ŹŹĮæČÜŅŗ£¬µĪČėĖįŠŌøßĆĢĖį¼ŲČÜŅŗ£¬ČōĶŹÉ«£¬Ö¤Ć÷ŗ¬ÓŠFe2+
C£®Č”ŹŹĮæČÜŅŗ£¬µĪČėĀČĖ®£¬ŌŁµĪČėKSCNČÜŅŗ£¬ČōĻŌŃŖŗģÉ«£¬Ö¤Ć÷ŌČÜŅŗÖŠŗ¬ÓŠFe2+
£Ø4£©¹¤³ĢŹ¦Óū“ÓÖĘŌģÓ”Ė¢µēĀ·°åµÄ·ĻĖ®ÖŠ»ŲŹÕĶ£¬²¢»ńµĆFeCl3ČÜŅŗ£¬Éč¼ĘČēĻĀ·½°ø£ŗ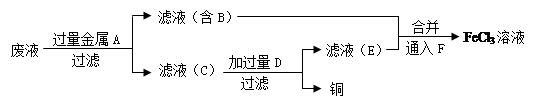
¢ŁĀĖŌüCµÄ»ÆѧŹ½ĪŖ ”£
¢Ś¼Ó¹żĮæD·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
¢ŪĶØČėF·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŹµŃéŹŅĄļ³£ÓƵÄøÉŌļ¼ĮÓŠ£ŗ¢ŁÉśŹÆ»Ņ£¬¢Ś¹ĢĢåĒāŃõ»ÆÄĘ£¬¢Ū±äÉ«¹č½ŗ£ŪÖ÷ŅŖ³É·ÖŹĒ¶žŃõ»Æ¹č£¬ŌŚĘäÖŠ²ōČėÉŁĮæµÄĪŽĖ®ĀČ»ÆīÜ(CoCl2)×÷ÖøŹ¾¼Į£Ż£¬¢ÜĪåŃõ»Æ¶žĮ×£¬¢ŻĪŽĖ®ĀČ»ÆøĘ£¬¢ŽÅØĮņĖį£¬¢ß¼īŹÆ»Ņ(Ö÷ŅŖ³É·ÖŹĒĒāŃõ»ÆÄĘ”¢Ńõ»ÆøĘ)µČ”£
£Ø1£©ÉĻŹöĪļÖŹÖŠ£¬ŹōÓŚ“æ¾»ĪļµÄŹĒ__________”£
A£®¢Ł¢Ś¢Ü B£®¢Ś¢Ü¢Ž C£®¢Ł¢Ś¢Ü¢Ż D£®Č«²æ
£Ø2£©ÉĻŹö¢Ś”¢¢Ü”¢¢Ż”¢¢ŽĖÄÖÖøÉŌļ¼ĮÖŠ£¬ĘäÖ÷ŅŖ»Æѧ³É·ÖĖłŹōµÄĄą±šŅĄ“ĪĪŖ______”¢________”¢________”¢______”££ØĢīŠņŗÅ£©
A£®Ėį B£®¼ī C£®ŃĪ D£®Ńõ»ÆĪļ
£Ø3£©¹č½ŗÖŠĪŽĖ®ĀČ»ÆīÜ(CoCl2)³ŹĄ¶É«£¬ĪüĖ®ŗó±äĪŖ·ŪŗģÉ«µÄCoCl2?6H2O£¬øƱä»Æ¹ż³ĢŹōÓŚ____________(Ģī”°ĪļĄķ±ä»Æ”±»ņ”°»Æѧ±ä»Æ”±)”£
£Ø4£©ĻĀĮŠĘųĢåÖŠ£¬ÄÜÓĆ¹ĢĢåĒāŃõ»ÆÄĘøÉŌļµÄŹĒ£Ø £©
A£®CO2 B£®HCl C£®NO2 D£®NH3 E£®NO
£Ø5£©ÉśŹÆ»Ņ³£ÓĆ×÷Ź³Ę·øÉŌļ¼Į£¬¾ĆÖĆŗóŅ׏§Č„øÉŌļÄÜĮ¦£¬ĘäŌŅņĪŖ_________________ (ÓĆ»Æѧ·½³ĢŹ½±ķŹ¾)
£Ø6£©ÉĻŹöøÉŌļ¼ĮÖŠ£¬½öÓŠÅØĮņĖįĪŖŅŗĢåøÉŌļ¼Į£¬ĻĀĮŠ¹ŲÓŚÅØĮņĖįµÄŠšŹöÕżČ·µÄŹĒ£Ø £©
A£®ÅØĮņĖį¾ßÓŠĪüĖ®ŠŌ£¬Ņņ¶ųÄÜŹ¹ÕįĢĒĢæ»Æ
B£®ÅØĮņĖįŌŚ³£ĪĀĻĀæÉŃøĖŁÓėĶʬ·“Ó¦·Å³ö¶žŃõ»ÆĮņĘųĢå
C£®ÅØĮņĖįĪŖŅŗĢåøÉŌļ¼Į£¬øÉŌļŠ§ĀŹøߣ¬ÄÜÓĆÓŚøÉŌļĖłÓŠµÄĘųĢå
D£®ÅØĮņĖįŌŚ³£ĪĀĻĀÄܹ»Ź¹Ģś”¢ĀĮµČ½šŹō¶Ū»Æ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
»Æ¹¤Éś²śÖŠ³£ÓƵ½”°ČżĖįĮ½¼ī”±£¬”°ČżĖį”±ÖøĻõĖį”¢ĮņĖįŗĶŃĪĖį£¬”°Į½¼ī”±ÖøÉÕ¼īŗĶ“æ¼ī”£
£Ø1£©“ÓĪļÖŹµÄ·ÖĄą½Ē¶Čæ“£¬²»Ē”µ±µÄŅ»ÖÖĪļÖŹŹĒ________£ØĢīĪļÖŹĆū³Ę£©”£
£Ø2£©”°ČżĖį”±Óė”°Į½¼ī”±Ö®¼äµÄ·“Ó¦£¬ČōÓĆ»Æѧ·½³ĢŹ½±ķŹ¾ÓŠĮłøö£ØĖį¹żĮæŹ±£© £¬ČōÓĆĄė×Ó·½³ĢŹ½±ķŹ¾Č“Ö»ÓŠĮ½øö£¬ĒėŠ“³öÕāĮ½øöĄė×Ó·½³ĢŹ½£ŗ£ØĖį¹żĮæŹ±£©_____________________________”¢______________________”£
£Ø3£©”°ČżĖį”±³£ÓĆÓŚČܽā½šŹōŗĶ½šŹōŃõ»ÆĪļ”£ĻĀĮŠæéד½šŹōŌŚ³£ĪĀŹ±ÄÜČ«²æČÜÓŚ×ćĮæÅØĻõĖįµÄŹĒ________”£
| A£®Au | B£®Cu |
| C£®Al | D£®Fe |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
(14·Ö)NH4Al(SO4)2ŹĒŹ³Ę·¼Ó¹¤ÖŠ×īĪŖæģ½ŻµÄŹ³Ę·Ģķ¼Ó¼Į£¬ÓĆÓŚ±ŗæ¾Ź³Ę·ÖŠ£»NH4HSO4ŌŚ·ÖĪöŹŌ¼Į”¢Ņ½Ņ©”¢µē×Ó¹¤ŅµÖŠÓĆĶ¾¹ć·ŗ”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)NH4Al(SO4)2æÉ×÷¾»Ė®¼Į£¬ĘäĄķÓÉŹĒ____________________________________________(ÓƱŲŅŖµÄ»ÆѧÓĆÓļŗĶĻą¹ŲĪÄ×ÖĖµĆ÷)”£
(2)ĻąĶ¬Ģõ¼žĻĀ£¬0.1 mol”¤L£1NH4Al(SO4)2ÖŠc(NH4+)________(Ģī”°µČÓŚ”±”¢”°“óÓŚ”±»ņ”°Š”ÓŚ”±)0.1 mol”¤L£1NH4HSO4ÖŠc(NH4+))”£
(3)ČēĶ¼ŹĒ0.1 mol”¤L£1ĖÄÖÖµē½āÖŹČÜŅŗµÄpHĖęĪĀ¶Č±ä»ÆµÄĶ¼Ļń”£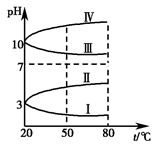
¢ŁĘäÖŠ·ūŗĻ0.1 mol”¤L£1NH4Al(SO4)2µÄpHĖęĪĀ¶Č±ä»ÆµÄĒśĻߏĒ________(ĢīŠ“ŠņŗÅ)£¬µ¼ÖĀpHĖęĪĀ¶Č±ä»ÆµÄŌŅņŹĒ____________________________£»
¢Ś20 ”ꏱ£¬0.1 mol”¤L£1NH4Al(SO4)2ÖŠ2c(SO42-)£c(NH4+))£3c(Al3£«)£½________”££Ø¼ĘĖć¾«Č·Öµ£©(4)ŹŅĪĀŹ±£¬Ļņ100 mL 0.1 mol”¤L£1 NH4HSO4ČÜŅŗÖŠµĪ¼Ó0.1 mol”¤L£1 NaOHČÜŅŗ£¬µĆµ½ČÜŅŗpHÓėNaOHČÜŅŗĢå»żµÄ¹ŲĻµĒśĻßČēĶ¼ĖłŹ¾£ŗ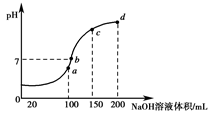
ŹŌ·ÖĪöĶ¼ÖŠa”¢b”¢c”¢dĖÄøöµć£¬Ė®µÄµēĄė³Ģ¶Č×ī“óŹĒ________µć£»ŌŚbµć£¬ČÜŅŗÖŠø÷Ąė×ÓÅضČÓɓ󵽊”µÄÅÅĮŠĖ³ŠņŹĒ______________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĻÖÓŠĻĀĮŠŹ®ÖÖĪļÖŹ£ŗ¢ŁH2 ¢ŚĀĮ ¢ŪCuO ¢ÜCO2 ¢ŻH2SO4 ¢ŽBa(OH)2 ¢ßŗģŗÖÉ«µÄĒāŃõ»ÆĢśŅŗĢå ¢ą°±Ė® ¢įĻ”ĻõĖį ¢āAl2(SO4)3
£Ø1£©°“ĪļÖŹµÄ·ÖĄą·½·ØĢīŠ“±ķøńµÄæհד¦£ŗ
| ·ÖĄą±ź×¼ | | Ńõ»ÆĪļ | | | µē½āÖŹ |
| ŹōÓŚøĆĄąµÄĪļÖŹ | ¢Ś | | ¢ą¢į | ¢ß | |
 H2O£¬øĆĄė×Ó·“Ó¦¶ŌÓ¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
H2O£¬øĆĄė×Ó·“Ó¦¶ŌÓ¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
½«×ćĮæµÄCO2²»¶ĻĶØČėKOH”¢Ba£ØOH£©2”¢KAlO2µÄ»ģŗĻČÜŅŗÖŠ£¬Éś³É³ĮµķÓėĶØČėCO2µÄĮæ¹ŲĻµæɱķŹ¾ĪŖ£Ø £© 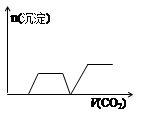
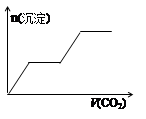
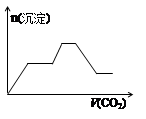
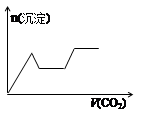
A B C D
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠĄė×Ó·½³ĢŹ½ÕżČ·µÄŹĒ£Ø £©
| A£®³ĪĒåŹÆ»ŅĖ®ÖŠµĪ¼ÓĻ”ĮņĖį£ŗCa(OH)2+2H+£½Ca2++2H2O |
| B£®ŹµŃéŹŅÓĆ“óĄķŹÆŗĶĻ”ŃĪĖįÕżČ·CO2:2H++CO32-= CO2”ü+H2O |
| C£®ĮņĖįĶČÜŅŗÓėĒāŃõ»Æ±µČÜŅŗ·“Ó¦£ŗBa2++SO42-=BaSO4”ż”£ |
| D£®¹żĮæµÄSO2ÓėNaOHČÜŅŗ·“Ó¦£ŗSO2+OH-=HSO3- |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com