
 2015Äź8ŌĀ12ŗŽӽüĪēŅ¹Ź±·Ö£¬Ģģ½ņ±õŗ£ŠĀĒųŅ»“¦¼Æ×°ĻäĀėĶ··¢Éś±¬ÕØ£®·¢Éś±¬ÕØµÄŹĒ¼Æ×°ĻäÄŚµÄŅ×Č¼Ņ×±¬ĪļĘ·£¬±¬ÕØ»š¹āÕšĢģ£¬²¢²śÉś¾Ž“óÄ¢¹½ŌĘ£®
2015Äź8ŌĀ12ŗŽӽüĪēŅ¹Ź±·Ö£¬Ģģ½ņ±õŗ£ŠĀĒųŅ»“¦¼Æ×°ĻäĀėĶ··¢Éś±¬ÕØ£®·¢Éś±¬ÕØµÄŹĒ¼Æ×°ĻäÄŚµÄŅ×Č¼Ņ×±¬ĪļĘ·£¬±¬ÕØ»š¹āÕšĢģ£¬²¢²śÉś¾Ž“óÄ¢¹½ŌĘ£®·ÖĪö £Ø1£©ŌŖĖŲµÄ·Ē½šŹōŠŌŌ½Ē棬µŚŅ»µēĄėÄÜŌ½“ó£¬ĘäÖŠµŚ¢ņA×唢¢õA×åÓÉÓŚ×īĶā²ćŹĒĪČ¶Ø½į¹¹£¬ĖłŅŌøßÓŚĶ¬ÖÜĘŚĻąĮŚŌŖĖŲ£»
£Ø2£©·Ö×ÓÖ®¼äÓŠĒā¼üŹ±£¬ĪļÖŹµÄČܽā¶ČŌö“ó£¬ÉÕ¼īÓÉÄĘĄė×ÓŗĶĒāŃõøł¹¹³É£¬S2-µÄ×īĶā²ćÓŠ18øöµē×Ó£¬øł¾ŻÄÜĮæ×īµĶæɏ銓³öĘ仳Ģ¬µē×ÓÅŲ¼Ź½£»
£Ø3£©ĻõĖįļ§ÖŠ£¬NO3-µÄµŖŌ×ӵļŪ²ćµē×Ó¶ŌŹżĪŖ$\frac{5+1}{2}$=3£¬¾Ż“ĖÅŠ¶ĻĮ¢Ģå¹¹ŠĶŗĶÖŠŠÄŌ×ÓµÄŌӻƹģµĄĄąŠĶ£»
£Ø4£©CN-ĖłÓėµŖĘų»„ĪŖµČµē×ÓĢ壬¾Ż“ĖÅŠ¶Ļ¦Š¼üŹż£¬-CNÖŠŗ¬ÓŠĮ½øöŌ×Ó”¢10øö¼Ūµē×Ó£¬¾Ż“ĖÅŠ¶ĻÓė-CN»„ĪŖµČµē×ÓĢåµÄ·Ö×Ó£¬Ä£·ĀĀČĘųÓė¶žŃõ»ÆĆĢµÄ·“Ó¦£¬øł¾ŻŌŖĖŲŹŲŗćæÉŠ“³öĒč»ÆÄĘ”¢¶žŃõ»ÆĆĢŗĶÅØĮņĖįŌŚ¼ÓČČĢõ¼žĻĀÖĘµĆ£ØCN£©2µÄ»Æѧ·½³ĢŹ½£»
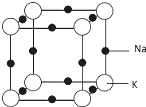
£Ø5£©øł¾Ż¾łĢÆ·ØæÉÖŖ¾§°ūÖŠÓŠÄĘŌ×ÓŹżĪŖ12”Į$\frac{1}{4}$=3£¬¼ŲŌ×ÓŹżĪŖ8”Į$\frac{1}{8}$=1£¬¾Ż“ĖČ·¶ØŗĻ½šµÄ»ÆѧŹ½£¬øł¾Ż¾§°ūĶ¼æÉÖŖ£¬ĆæøöK Ō×ÓÖÜĪ§ÓŠ6øöÄĘŌ×Ó£»øł¾Ż¾§°ūµÄ½į¹¹æÉÖŖ£¬¾§°ūµÄ±ß³¤ĪŖÄĘŌ×ÓŗĶ¼ŲŌ×ÓµÄÖ±¾¶Ö®ŗĶ£¬¾§ĢåµÄæÕ¼äĄūÓĆĀŹĪŖ$\frac{ÄĘŌ×ÓÓė¼ŲŌ×ÓµÄĢå»żÖ®ŗĶ}{¾§µÄĢå»ż}$”Į100%£¬¾Ż“Ė“šĢā£®
½ā“š ½ā£ŗ£Ø1£©ŌŖĖŲµÄ·Ē½šŹōŠŌŌ½Ē棬µŚŅ»µēĄėÄÜŌ½“ó£¬ĘäÖŠµŚ¢ņA×唢¢õA×åÓÉÓŚ×īĶā²ćŹĒĪČ¶Ø½į¹¹£¬ĖłŅŌøßÓŚĶ¬ÖÜĘŚĻąĮŚŌŖĖŲ£¬ĖłŅŌNH4NO3”¢NaCNĮ½ÖÖĪļÖŹµÄŌŖĖŲÖŠµŚŅ»µēĄėÄÜ×ī“óµÄŹĒN£¬ŅņĪŖµŖŌ×Ó×īĶā²ćp¹ģµĄ“¦ÓŚ°ėĀś×“Ģ¬£¬ŹĒŅ»ÖÖĪČ¶Ø½į¹¹£¬
¹Ź“š°øĪŖ£ŗN£»µŖŌ×Ó×īĶā²ćp¹ģµĄ“¦ÓŚ°ėĀś×“Ģ¬£¬ŹĒŅ»ÖÖĪČ¶Ø½į¹¹£»
£Ø2£©¼×ĖįÓėĖ®ŠĪ³ÉĒā¼ü£¬ĖłŅŌ¶ž¼×»ł¶žĮņŗĶ¼×ĖįÖŠÖŠČܽā¶Č½Ļ“óµÄŹĒ¼×Ėį£¬ÉÕ¼īÓÉÄĘĄė×ÓŗĶĒāŃõøł¹¹³É£¬ĖłŅŌÉÕ¼īŹĒĄė×Ó¾§Ģ壬S2-µÄ×īĶā²ćÓŠ18øöµē×Ó£¬Ę仳Ģ¬µē×ÓÅŲ¼Ź½ĪŖ1s2s22p63s23p6£¬
¹Ź“š°øĪŖ£ŗ¼×Ėį£»¼×ĖįÓėĖ®ŠĪ³ÉĒā¼ü£»Ąė×Ó¾§Ģ壻1s2s22p63s23p6£»
£Ø3£©ĻõĖįļ§ÖŠ£¬NO3-µÄµŖŌ×ӵļŪ²ćµē×Ó¶ŌŹżĪŖ$\frac{5+1}{2}$=3£¬ĖłŅŌNO3-Į¢Ģå¹¹ŠĶĪŖĘ½ĆęČż½ĒŠĪ£¬ÖŠŠÄŌ×ÓµŖŌ×ÓµÄŌӻƹģµĄĄąŠĶsp2£¬
¹Ź“š°øĪŖ£ŗĘ½ĆęČż½ĒŠĪ£»sp2£»
£Ø4£©CN-ĖłÓėµŖĘų»„ĪŖµČµē×ÓĢ壬ĆæøöµŖ·Ö×ÓÖŠÓŠ2øö¦Š¼ü£¬ĖłŅŌ1mol»ÆŗĻĪļNaCNÖŠCN-Ėłŗ¬µÄ¦Š¼üŹżĪŖ2NA£¬-CNÖŠŗ¬ÓŠĮ½øöŌ×Ó”¢10øö¼Ūµē×Ó£¬Óė-CN»„ĪŖµČµē×ÓĢåµÄ·Ö×ÓÓŠCO”¢N2£¬Ēč»ÆÄĘ”¢¶žŃõ»ÆĆĢŗĶÅØĮņĖįŌŚ¼ÓČČĢõ¼žĻĀÖĘµĆ£ØCN£©2µÄ»Æѧ·½³ĢŹ½ĪŖ2NaCN+MnO2+2H2SO4$\frac{\underline{\;\;”÷\;\;}}{\;}$ £ØCN£©2+Na2SO4+MnSO4+2H2O£¬
¹Ź“š°øĪŖ£ŗ2NA£»CO”¢N2£»2NaCN+MnO2+2H2SO4$\frac{\underline{\;\;”÷\;\;}}{\;}$ £ØCN£©2+Na2SO4+MnSO4+2H2O£»
£Ø5£©øł¾Ż¾łĢÆ·ØæÉÖŖ¾§°ūÖŠÓŠÄĘŌ×ÓŹżĪŖ12”Į$\frac{1}{4}$=3£¬¼ŲŌ×ÓŹżĪŖ8”Į$\frac{1}{8}$=1£¬ĖłŅŌŗĻ½šµÄ»ÆѧŹ½ĪŖKNa3£¬øł¾Ż¾§°ūĶ¼æÉÖŖ£¬ĆæøöK Ō×ÓÖÜĪ§ÓŠ6øöÄĘŌ×Ó£¬ĖłŅŌ¾§°ūÖŠK Ō×ÓµÄÅäĪ»ŹżĪŖ6£¬¾§°ūÖŠÄĘŌ×ÓŗĶ¼ŲŌ×ÓĢå»żÖ®ŗĶĪŖ$\frac{4}{3}¦Š£Ø18{6}^{3}”Į3+22{7}^{3}£©$£¬øł¾Ż¾§°ūµÄ½į¹¹æÉÖŖ£¬¾§°ūµÄ±ß³¤ĪŖÄĘŌ×ÓŗĶ¼ŲŌ×ÓµÄÖ±¾¶Ö®ŗĶĪŖ2”Į£Ø186pm+227pm£©£¬ĖłŅŌ¾§°ūµÄĢå»żĪŖ£Ø2”Į186pm+2”Į227pm£©3£¬¾§ĢåµÄæÕ¼äĄūÓĆĀŹĪŖ$\frac{ÄĘŌ×ÓÓė¼ŲŌ×ÓµÄĢå»żÖ®ŗĶ}{¾§µÄĢå»ż}$”Į100%=$\frac{\frac{4}{3}¦Š£Ø18{6}^{3}”Į3+22{7}^{3}£©}{£Ø186”Į2+227”Į2£©^{3}}$”Į100%£¬
¹Ź“š°øĪŖ£ŗKNa3£»6£»$\frac{\frac{4}{3}¦Š£Ø18{6}^{3}”Į3+22{7}^{3}£©}{£Ø186”Į2+227”Į2£©^{3}}$”Į100%£®
µćĘĄ ±¾ĢāŹĒ¶ŌĪļÖŹ½į¹¹ÓėŠŌÖŹµÄ漲飬ĪŖøßĘµæ¼µć£¬²ąÖŲӌѧɜµÄ·ÖĪöÄÜĮ¦ŗĶ¼ĘĖćÄÜĮ¦µÄ漲飬±¾ĢāÉę¼°½į¹¹ŠŌÖŹĪ»ÖĆ¹ŲĻµ”¢ŗĖĶāµē×ÓÅŲ¼”¢·Ö×Óæռ乹ŠĶ”¢µČµē×ÓĢ唢¾§°ū¼ĘĖćµČ£¬ÄѶČÖŠµČ£¬×¢Ņā»ł“”ÖŖŹ¶µÄĄķ½āÕĘĪÕ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
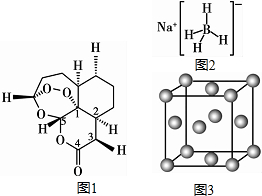 ĻÖÓŠH”¢C”¢B”¢0”¢N”¢Na”¢CuµČĘßÖÖŌŖĖŲ£¬æÉŠĪ³É¶ąÖÖĪļÖŹ£®2015Äź10ŌĀÖŠ¹śŅ©Ń§¼ŅĶĄßĻßĻŅņ·¢ĻÖĒąŻļĖŲ£Øŗ¬H”¢C”¢OŌŖĖŲ£©¶ų»ńµĆŵ±“¶ūÉśĄķŅ½Ń§½±£¬ĒąŻļĖŲ£Ø C15H22O5£©µÄ½į¹¹
ĻÖÓŠH”¢C”¢B”¢0”¢N”¢Na”¢CuµČĘßÖÖŌŖĖŲ£¬æÉŠĪ³É¶ąÖÖĪļÖŹ£®2015Äź10ŌĀÖŠ¹śŅ©Ń§¼ŅĶĄßĻßĻŅņ·¢ĻÖĒąŻļĖŲ£Øŗ¬H”¢C”¢OŌŖĖŲ£©¶ų»ńµĆŵ±“¶ūÉśĄķŅ½Ń§½±£¬ĒąŻļĖŲ£Ø C15H22O5£©µÄ½į¹¹²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 æĘѧ¼ŅĶعżXÉäĻßĶĘ²āµØ·ÆÖŠ¼Čŗ¬ÓŠÅäĪ»¼ü£¬ÓÖŗ¬ÓŠĒā¼ü£¬Ęä½į¹¹Ź¾ŅāĶ¼æɼņµ„±ķŹ¾ČēĻĀ£¬ĘäÖŠÅäĪ»¼üŗĶĒā¼ü¾ł²ÉÓĆŠéĻß±ķŹ¾£®
æĘѧ¼ŅĶعżXÉäĻßĶĘ²āµØ·ÆÖŠ¼Čŗ¬ÓŠÅäĪ»¼ü£¬ÓÖŗ¬ÓŠĒā¼ü£¬Ęä½į¹¹Ź¾ŅāĶ¼æɼņµ„±ķŹ¾ČēĻĀ£¬ĘäÖŠÅäĪ»¼üŗĶĒā¼ü¾ł²ÉÓĆŠéĻß±ķŹ¾£® £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ō×Ó°ė¾¶ | B£® | Ēā»ÆĪļµÄĪČ¶ØŠŌ | C£® | µē×Ó²ćŹż | D£® | µ„ÖŹµÄČŪ·Šµć |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŹÆÓĶĮŃ½ā”¢ĆŗµÄĘų»Æ”¢ŗ£Ė®ÖĘĆ¾µČ¹ż³ĢÖŠ¶¼°üŗ¬»Æѧ±ä»Æ | |
| B£® | ŌŚ¼“½«µ½Ą“µÄŠĀÄÜŌ“Ź±“ś£¬ŗĖÄÜ”¢Ģ«ŃōÄÜ”¢ĒāÄܽ«³ÉĪŖÖ÷ŅŖÄÜŌ“ | |
| C£® | øß“æ¶ČµÄ¶žŃõ»Æ¹č¹ć·ŗÓĆÓŚÖĘ×÷¹āµ¼ĻĖĪ¬£¬¹āµ¼ĻĖĪ¬ÓöĒæĖį”¢Ēæ¼ī¶¼»į”°¶ĻĀ·”± | |
| D£® | Ó²Ö¬ĖįøŹÓĶõ„ŌŚ¼īŠŌĢõ¼žĻĀµÄĖ®½āŹōÓŚŌķ»Æ·“Ó¦£¬ŅŅĖįŅŅõ„ŌŚ¼īŠŌĢõ¼žĻĀµÄĖ®½ā²»ŹōÓŚŌķ»Æ·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĶʶĻĢā
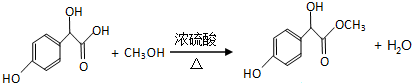
 £®
£®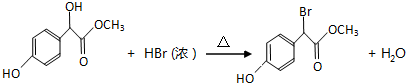
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | ¼×ĶéµÄĒņ¹÷Ä£ŠĶ£ŗ | |
| B£® | ŅŅČ²µÄ½į¹¹¼ņŹ½£ŗCH=CH | |
| C£® | 3-¼×»ł-1-¶”Ļ©µÄ½į¹¹¼ņŹ½£ŗ£ØCH3£©2CHCH=CH2 | |
| D£® | ōĒ»łµÄµē×ÓŹ½£ŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ł¢Ś | B£® | ¢Ś¢Ū | C£® | ¢Ū¢Ü | D£® | ¢Ł¢Ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | X2ĪŖ0.2mol/L | B£® | Y2ĪŖ0.35mol/L | ||
| C£® | XYĪŖ0.25mol/L | D£® | X2£¬Y2£¬XY×ÜÅضČĪŖ0.6mol/L |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com