
��14�֣�������(��Ҫ�ɷ�ΪAl2O3��SiO2��Fe2O3)����ȡ��������ԭ�ϡ���ȡ�������Ĺ����������£�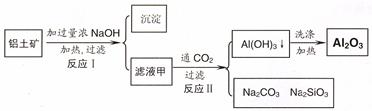
��1����Һ����Ҫ�ɷ��ǣ�д��ѧʽ�� ������������������������
��2��д����Ӧ II �����ӷ���ʽ��
��3����Ϸ�ӦII���ж�������������� ( H+) ����������ǿ������˳���� ������ĸ��ţ�
A��AlO2�D B��OH�D C��SiO32�D
��4��ȡ��Һ������������������ᣬ���ˣ����ö��Ե缫�������Һ���������������������ȫ���ݳ��������������г������ɣ���������ʧ��������ʧ��ԭ��������ӷ���ʽ��ʾΪ��
��5��ȡ ��4�� ����Ժ����Һ 10.0 mL��������������Һ��ֻ�������ֵ����ʵ���Ũ�ȵļ������ʣ���������μ���0.100 mol? L��1������Һ��������50.0mL ������Һʱ�����ɵij���ǡ���ܽ⡣
�ټ���50.0mL ������Һ���������ӷ�Ӧ���Ⱥ�˳������Ϊ��
���뻭�����ɳ��������ʵ����������������Ĺ�ϵͼ��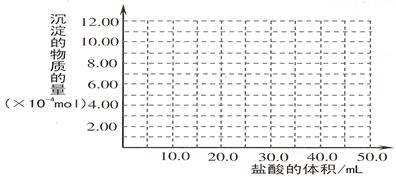
�š�NaOH��NaAlO2��Na2SiO3
�ơ�CO2+2OH��==CO32��+2H2O CO2��2H2O +2 AlO2��==2Al(OH)3��+HCO3��
�ǡ�b��a��c �ȡ�Al(OH)3 +OH����AlO2��+2H2O
�� �� H����OH��==H2O AlO2-��H��+H2O =Al(OH)3�� Al(OH)3��3H��=Al3����3H2O
��ͼ����������ʾ��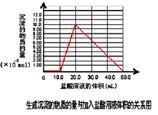
���������������1���������м��������NaOH��Һ�����е�Al2O3��SiO2������Ӧ�õ�NaAlO2��Na2SiO3������NaOH��Һ��������Һ����Ҫ�ɷ���NaAlO2��Na2SiO3��NaOH�������ԵĹ�����Fe2O3����2������NaAlO2��Na2SiO3��NaOH����Һ��ͨ�����CO2��������ӦCO2+2OH��=CO32��+2H2O ��CO2��2H2O +2AlO2��==2Al(OH)3��+HCO3�����õ�Al(OH)3��������3��H2O���������ʣ�Al(OH)3���������ʣ�H2SiO3�����ᡣ������������� ( H+) ����������ǿ������˳����b��a��c����4������NaAlO2��Na2SiO3��NaOH�ļ���������ᷢ����Ӧ�õ�AlCl3��NaCl��H2SiO3����ҺΪAlCl3��NaCl�Ļ����Һ���ö��Ե缫��⣬������������Ӧ��2H++2e-=H2��,�����ƻ��˸�����ˮ�ĵ���ƽ�⣬��Һ��OH-��Ũ������OH-����Һ�е�Al3+������Ӧ�γ�Al(OH)3������������������Ӧ��2Cl-��2e-=Cl2��������Һ�ʼ���ʱ���ַ�����Ӧ��Al(OH)3+ OH-= AlO2-+ 2H2O�����������ܽ����ʧ����5���ټ���50.0mL ������Һ���������ӷ�Ӧ���Ⱥ�˳����H����OH��=H2O ��AlO2-��H��+H2O =Al(OH)3�� Al(OH)3��3H��=Al3����3H2O �� ���ڶ��ߵ����ʵ�����ȣ��������Ƕ�����0.100 mol/L��0.01L=0.001mol��NaOH��HCl��Ӧ����10mlHCl; NaAlO2��HCl��Ӧ�γ�Al(OH)3��������10mlHCl;�ܽ�Al(OH)3��������30mlHCl.ͼ���ͼʾ:
���㣺����Ԫ���뻯��������ʡ������ķ��롢���ӷ���ʽ����д��n(HCl)������Ĺ�ϵͼ���֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����Mg��Al��ɵ�һ�������Ļ����Ͷ��500 mL ϡ�����У�����ȫ���ܽⲢ�������塣����Ӧ��ȫ����������Һ�м���NaOH��Һ�����ɳ��������ʵ��������NaOH��Һ�������ϵ����ͼ��ʾ��������˵����ȷ����
| A��������Mg��Al��ɵĻ���������Ϊ8g |
| B����������ʵ���Ũ��Ϊ1 mol��L��1 |
| C�����ɵ�H2�ڱ�״���µ����Ϊ11.2L |
| D��NaOH��Һ�����ʵ���Ũ��Ϊ3.75 mol��L��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ͼ��ʾ������ת����ϵ�У���Ӧ�����Ͳ���������δȫ���г�����X���ʿ��������ں����У�A��BΪ�������嵥�ʣ�BΪ����ɫ���壬I��LΪ�����Ľ������ʣ�GΪ���ɫ���ʡ���ش��������⣺
��1��X��ѧʽΪ �� ��2��C��ѧʽΪ ��
��3����Ӧ�ٵ����ӷ���ʽΪ ��
��4����Ӧ�ڵ����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(12��)ij��ѧ��ȤС����ֻ������������ͭ�Ĺ�ҵ������ȡ�������Ȼ�����Һ���̷�����(FeSO4��7H2O)�͵������壬��̽����ҵ���ϵ������á���ʵ�鷽�����£�
��1��д���Ͻ����ռ���Һ��Ӧ�����ӷ���ʽ������������������������������������
��2������ҺA��AlCl3��Һ��;���Тٺ͢����֣�����Ϊ�Ϻ�����;���������ǣ��� �������������� �� ���� ��������
��3����ҺE�������ڿ�����һ��ʱ�����Һ�е������ӳ���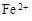 ��
��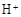 �⣬�����ܴ���������������������Ԫ�ط��ű�ʾ����
�⣬�����ܴ���������������������Ԫ�ط��ű�ʾ����
��4��������Fͨ������;����ȡ��������;������ȣ�;�������Ծ��е������ŵ��ǣ�
�������������������������������� ������������ �������� �������� ������������
��5��;���ܷ�����Ӧ�Ļ�ѧ����ʽΪ������������������ �� ������������������
��6��ʵ���Ҵ�CuSO4��Һ��ȡ��������������������Ũ������ȴ�ᾧ������������������Ȼ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��12�֣�������������Ч���ٶ���������ŷš�ʵ�����÷�ú�ң���Ҫ��Al2O3��SiO2�ȣ��Ʊ���ʽ������[Al2(SO4)x(OH)6��2x]��Һ�����������������о���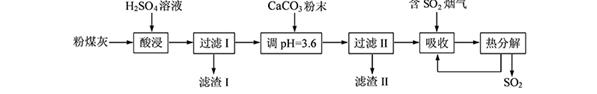
��1�����ʱ��Ӧ�Ļ�ѧ����ʽΪ �����������Ҫ�ɷ�Ϊ ���ѧʽ����
��2����CaCO3������Һ��pH��3.6����Ŀ�����к���Һ�е��ᣬ��ʹAl2(SO4)3ת��ΪAl2(SO4)x(OH)6��2x�����������Ҫ�ɷ�Ϊ ���ѧʽ��������Һ��pHƫ�ߣ����ᵼ����Һ����Ԫ�صĺ������ͣ���ԭ���� �������ӷ���ʽ��ʾ����
��3�����������о���ȫ�ȷֽ�ų���SO2������С�����յ�SO2��������Ҫԭ���� ��������SO2ǰ����Һ��ȣ��ȷֽ��ѭ�����õ���Һ��pH�� �����������С�����䡱����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��12�֣�ij�о���ѧϰС���������Ѽ�����Ϣ���ء��ơ��ơ�þ�Ȼ��ý�������CO2������ȼ�ա����Ƕ�����CO2������ȼ�ս���������ʵ�飺
| �������� | ʵ������ |
| ������IJ���ȼ�ճ���ȼ�յ���Ѹ�� ���뵽ʢ��װ��CO2�ļ���ƿ�� | ����ʢ��CO2�ļ���ƿ�м���ȼ�� |
| ��Ӧ����ȴ | ����ƿ���ź�ɫ������ƿ���ϸ����а�ɫ ���� |
| ʵ�鲽�� | ʵ������ |
| ��ȡ������ɫ�������Թ��У���������ˮ������Ʒȫ ������ˮ�������м��������CaCl2��Һ | ���ְ�ɫ���� |
| �ھ���Ƭ�̣�ȡ�ϲ���Һ���Թ��У��μ���ɫ��̪��Һ | ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��15�֣�����ͭ�ڻ�����ũҵ�����кܹ㷺���ô���ij��ѧ��ȤС��������ϣ������ֲ�ͬ��ԭ����ȡ����ͭ��
��ʽһ��һ�ֺ�ͭ�Ŀ�ʯ�����ȸʯ��ۣ�����ͭ��̬ΪCuCO3��Cu(OH)2��CuSiO3��2H2O������SiO2��FeCO3��Fe2O3��Al2O3�����ʣ��������ֿ�ʯΪԭ����ȡ����ͭ�Ĺ�����������ͼ��
��ش��������⣺
����ɲ������ϡ������CuSiO3��2H2O������Ӧ�Ļ�ѧ����ʽ
CuSiO3��2H2O+H2SO4=CuSO4 +________+H2O��
�Ʋ���ڵ�����ҺpHѡ�õ�����Լ���__________________
A. CuO B. MgO C. FeCO3 D NH3��H2O
���й��������↑ʼ��������ȫ������pH���±���
| �������� | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Cu(OH)2 |
| ��ʼ������pH | 3.3 | 1.5 | 6.5 | 4.2 |
| ������ȫ��pH | 5.2 | 3.7 | 9.7 | 6.7 |

 R2Cu���л��ࣩ+ 2H����ˮ�ࣩ
R2Cu���л��ࣩ+ 2H����ˮ�ࣩ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������(��Ҫ��Fe2O3��SiO2��Al2O3��MgO������)�Ʊ��������Ĺ����������£�
��1���������������Ҫ�ʵ�������Ŀ���ǣ���������Ľ����ʣ��� ��
��2������ԭ���ǽ�Fe3��ת��ΪFe2����ͬʱFeS2������ΪSO42�����÷�Ӧ�����ӷ���ʽΪ
��
��3��Ϊ�ⶨ��������������Һ��Fe3�������Կ��Ƽ���FeS2������ʵ�鲽��Ϊ��
ȷ��ȡһ���������������Һ����ƿ�У�����HCl���Թ���SnCl2���ټ�HgCl2��ȥ������SnCl2���Զ�����������Ϊָʾ������K2Cr2O7����Һ�ζ����йط�Ӧ����ʽ���£�
2Fe3����Sn2����6Cl����2Fe2����SnCl62����
Sn2����4Cl����2HgCl2��SnCl62����Hg2Cl2����
6Fe2����Cr2O72����14H����6Fe3����2Cr3����7H2O��
����SnCl2����������ⶨ��Fe3���� (�ƫ�ߡ�����ƫ�͡��������䡱����ͬ)��
��������HgCl2����ⶨ��Fe3���� ��
��4���ٿ�ѡ�� (���Լ�)������Һ�к���Fe3+������Fe3+��ԭ����
(�����ӷ�Ӧ����ʽ��ʾ)��
����֪����������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Al(OH)3 | Fe(OH)2 | Mg(OH)2 | Mn(OH)2 |
| ��ʼ���� | 2.7 | 3.8 | 7.5 | 9.4 | 8.3 |
| ��ȫ���� | 3.2 | 5.2 | 9.7 | 12.4 | 9.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
1862�꣬����ʱ��ѧ������ά�����˰���Ƽ1926�꣬�ҹ���ѧ�Һ�°�����
��Ϊ����°��Ƽ��Ҳ�������Ƽ�������Ƽ���������̿ɼ�Ҫ��ʾ����ͼ��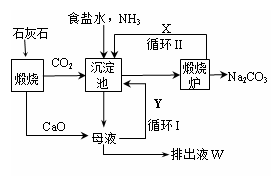

����������� �����Ƽ��������
��1�����������ͨ��CO2�Ͱ���ʱ��Ӧ��ͨ�백����ԭ���� ��
��2���������з�����Ӧ�Ļ�ѧ��Ӧ����ʽ�� �ӳ������з�������IJ����� ��
��3�������������ʾ��ͼ�е�Y�� ����ԭ�ϵ���Ʒ������ܷ�Ӧ�����û�ѧ����ʽ��ʾ����дΪ ��
��4�������Ƽ�д���Һ����ȡ�Ȼ�茶���Ĺ����Ʋ⣬���ý�����ȷ�� ��ѡ���ţ���
a������ʱ�Ȼ�淋��ܽ�ȱ��Ȼ���С
b��ͨ�백��������NH4+��Ũ�ȣ�ʹ�Ȼ�笠�������
c������ʳ��ϸ�������Na+��Ũ�ȣ� ʹNaHCO3�ᾧ����
d��ͨ�백����ʹNaHCO3ת��ΪNa2CO3�����������NH4Cl����
��5�������Ƽ����ڰ�����Ȼ��������ʴ�70%��ߵ�90%���ϣ���Ҫ�������ѭ���������Ƽ����һ���ŵ��� ��
��6����Ʒ�����к���̼�����ƣ������ü��ȷֽ�ķ����ⶨ��Ʒ�д����������������֪��Ʒ����Ϊag���������������ٸı�ʱ����Ϊbg�������������Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com