
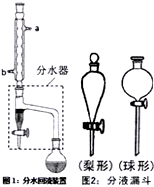
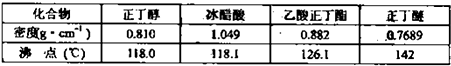
ĻĀĮŠ¹ŲÓŚŹµŃéµÄĖµ·ØÕżČ·µÄŹĒ
A£®°Ń40g NaOH¹ĢĢå·ÅČė1000mlĖ®ÖŠ£¬¼“æÉÅäÖĘ1.00 mol/L µÄNaOHČÜŅŗ
B£®ŹµŃéŹŅÅäÖĆ1 mol/LµÄŃĪĖį250ml£¬Ö»ŠčŅŖĶŠÅĢĢģĘ½”¢½ŗĶ·µĪ¹Ü”¢250mlČŻĮæĘ攢ÉÕ±
C£®ŌŚÅäÖĆŅ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄKOHČÜŅŗŹ±£¬ČŻĮæĘæĻ“¾»ŗóĪ“øÉŌļ”¢¶ØČŻŹ±ŃöŹÓ¹Ū²ģ¾łæÉŅŌŌģ³ÉŹµŃé½į¹ūĘ«µĶ
D£®ŹµŃéŹŅŠčŅŖ480ml0.1 mol/L µÄĮņĖįĶČÜŅŗ£¬æÉŅŌÓĆ500mlµÄČŻĮæĘæÅäÖĆ£¬ŠčŅŖ³ĘČ”µØ·Æ12.5g
E£®ÖʱøFe(OH)3½ŗĢåŹĒ½«±„ŗĶFeCl3ČÜŅŗµĪČė·ŠĢŚµÄÕōĮóĖ®ÖŠ£¬¼ĢŠųÖó·ŠÖĮČÜŅŗ³ŹŗģŗÖÉ«
F£®ĶعżÉųĪöæÉŅŌ³żČ„µķ·ŪČÜŅŗÖŠµÄĀČ»ÆÄĘ£¬Ķعż¶”“ļ¶ūĻÖĻóæÉŅŌĒų±šFe(OH)3½ŗĢåŗĶFeCl3ČÜŅŗ
G£®ĪŖĢįøßøßĆĢĖį¼ŲµÄŃõ»ÆÄÜĮ¦£¬æÉŅŌÓĆŃĪĖį½ųŠŠĖį»Æ
H£®½«¾ßÓŠĘư׊ŌµÄCl2ŗĶSO2µČĪļÖŹµÄĮæĶØČėĖ®ÖŠ£¬ČÜŅŗ¼øŗõƻӊĘÆ°×ÄÜĮ¦

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
 ŹµŃéŹŅÖʱøŅŅĖįÕż¶”õ„µÄ»Æѧ·½³ĢŹ½£ŗ
ŹµŃéŹŅÖʱøŅŅĖįÕż¶”õ„µÄ»Æѧ·½³ĢŹ½£ŗ| ÅØH2SO4 |
| ”÷ |
| ÅØH2SO4 |
| ”÷ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010-2011ѧğ½ĖÕŹ”Ģ©ÖŻŹŠøßČżµŚŅ»“ĪÄ£Äāæ¼ŹŌ»Æѧ¾ķ ĢāŠĶ£ŗŹµŃéĢā
£Ø12·Ö£©±¾Ģā°üĄØA”¢BĮ½Š”Ģā£¬·Ö±š¶ŌÓ¦ÓŚ”°ĪļÖŹ½į¹¹ÓėŠŌÖŹ”±ŗĶ”°ŹµŃé»Æѧ”±Į½øöŃ”ŠŽÄ£æéµÄÄŚČŻ£¬ĒėŃ”ŌńĘäÖŠ”ŖĢā£¬²¢ŌŚĻąÓ¦µÄ“šĢāĒųÓņ×÷“š”£ČōĮ½Ģā¶¼×ö£¬Ōņ°“AĢāĘĄ·Ö”£
A£®Ć¾”¢ĶµČ½šŹōĄė×ÓŹĒČĖĢåÄŚ¶ąÖÖĆøµÄøØŅņ×Ó”£¹¤ŅµÉĻ“Óŗ£Ė®ÖŠĢįČ”Ć¾Ź±£¬ĻČÖʱøĪŽĖ®ĀČ»ÆĆ¾£¬Č»ŗó½«ĘäČŪČŚµē½ā£¬µĆµ½½šŹōĆ¾”£
£Ø1£©Ēė²Īæ¼ĻĀŹöŹż¾ŻĢīæÕŗĶ»Ų“šĪŹĢā£ŗ

¹¤ŅµÉĻ³£ÓƵē½āČŪČŚMgCl2µÄ·½·ØÉś²ś½šŹōĆ¾£¬µē½āAl2O3Óė±ł¾§ŹÆČŪČŚ»ģŗĻĪļµÄ·½·ØÉś²śĀĮ”£²»ÓƵē½āMgOµÄ·½·ØÉś²śĆ¾µÄŌŅņ £»²»ÓƵē½āAlCl3µÄ·½·ØÉś²śĀĮµÄŌŅņ ”£
£Ø2£©2001ÄźŌų±ØµĄ£¬ÅšĆ¾»ÆŗĻĪļĖ¢ŠĀĮĖ½šŹō»ÆŗĻĪļ³¬µ¼ĪĀ¶ČµÄ×īøß¼ĒĀ¼”£øĆ»ÆŗĻ¾§Ģå½į¹¹ÖŠµÄ¾§°ūČēĶ¼ĖłŹ¾”£Ć¾Ō×Ó¼äŠĪ³ÉÕżĮłĄāÖł£¬ĮłøöÅšŌ×ÓĪ»ÓŚĄāֳČ”£ŌņøĆ»ÆŗĻĪļµÄ»ÆѧŹ½æɱķŹ¾ĪŖ ”£

£Ø3£©Š“³öCu+µÄŗĖĶāµē×ÓÅŲ¼Ź½ ”£
£Ø4£©ĶłĮņĖįĶČÜŅŗÖŠ¼ÓČė¹żĮæ°±Ė®£¬æÉÉś³É[Cu£ØNH3£©4]2+ÅäĄė×Ó”£ŅŃÖŖNF3ÓėNH3µÄæռ乹ŠĶ¶¼ŹĒČż½Ē׶ŠĪ£¬µ«NF3²»Ņ×ÓėCu2+ŠĪ³ÉÅäĄė×Ó£¬ĘäŌŅņŹĒ ”£
£Ø5£©Ä³ąßöĶŖĄąŅ©Īļ£ØÖŠŠÄĄė×ÓŹĒCu2£«£©½į¹¹ČēĻĀĶ¼£¬¹ŲÓŚøĆŅ©ĪļµÄĖµ·ØÕżČ·µÄŹĒ ”£

A£®ÖŠŠÄĄė×ÓCu2+µÄÅäĪ»ŹżŹĒ5
B£®NŌ×Ó¾ł²ÉÓĆsp2ŌÓ»Æ
C£®“ęŌŚÅäĪ»¼ü”¢¼«ŠŌ¹²¼Ū¼üŗĶ·Ē¼«ŠŌ¹²¼Ū¼ü
D£®ČŪµćŗÜøߣ¬Ó²¶ČŗÜ“ó
B£®Ä³»Æѧъ¾æŠŌѧĻ°Š”×éĪŖĢ½¾æÄ³Ę·ÅĘ»ØÉśÓĶÖŠ²»±„ŗĶÖ¬·¾ĖįµÄŗ¬Į棬½ųŠŠĮĖČēĻĀŹµŃé£ŗ
²½ÖčI£ŗ³ĘČ”0£®4 g»ØÉśÓĶѳʷ£¬ÖĆÓŚĮ½øöøÉŌļµÄµāĘæ£ØČēĶ¼£©ÄŚ£¬¼ÓČė10 mLĖÄĀČ»ÆĢ¼£¬ĒįĒįŅ”¶ÆŹ¹ÓĶČ«²æČܽā”£ĻņµāĘæÖŠ¼ÓČė25£®00 mLŗ¬0£®01 mol IBrµÄĪŽĖ®ŅŅĖįČÜŅŗ£¬øĒŗĆĘæČū£¬ŌŚ²£Į§ČūÓėĘææŚÖ®¼äµĪ¼ÓŹżµĪ10£„µā»Æ¼ŲČÜŅŗ·ā±Õ·ģĻ¶£¬ŅŌĆāIBrµÄ»Ó·¢ĖšŹ§”£

²½ÖčII£ŗŌŚ°µ“¦·ÅÖĆ30 min£¬²¢²»Ź±ĒįĒįŅ”¶Æ”£30 minŗ󣬊”ŠÄµŲ“ņæŖ²£Į§Čū£¬ÓĆŠĀÅäÖʵÄ10£„
µā»Æ¼Ų10 mLŗĶÕōĮóĖ®50 mL°Ń²£Į§ČūŗĶĘæ¾±ÉĻµÄŅŗĢå³åĻ“ČėĘæÄŚ”£
²½Öč¢ó£ŗ¼ÓČėÖøŹ¾¼Į£¬ÓĆ0£®1 mol”¤L£1Įņ“śĮņĖįÄĘČÜŅŗµĪ¶Ø£¬ÓĆĮ¦Õńµ“µāĘ棬ֱÖĮÖÕµć”£
²ā¶Ø¹ż³ĢÖŠ·¢ÉśµÄĻą¹Ų·“Ó¦ČēĻĀ£ŗ
¢Ł
¢ŚIBr£«KI=I2+KBr
¢ŪI2£«2S2O32£=2I££«S4O62£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŅŃÖŖĀ±ĖŲ»„»ÆĪļIBrµÄŠŌÖŹÓėĀ±ĖŲµ„ÖŹĄąĖĘ£¬ŹµŃéÖŠ×¼Č·ĮæČ”IBrČÜŅŗӦєÓƵÄŅĒĘ÷ŹĒ £¬µāĘæ²»øÉŌļ»į·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
£Ø2£©²½Öč¢ņÖŠµāĘæŌŚ°µ“¦·ÅÖĆ30 min£¬²¢²»Ź±ĒįĒįŅ”¶ÆµÄŌŅņŹĒ ”£
£Ø3£©²½Öč¢óÖŠĖł¼ÓÖøŹ¾¼ĮĪŖ £¬µĪ¶ØÖÕµćµÄĻÖĻó ”£
£Ø4£©·“Ó¦½įŹųŗó“ÓŅŗĢå»ģŗĻĪļÖŠ»ŲŹÕĖÄĀČ»ÆĢ¼£¬ĖłŠč²Ł×÷ÓŠ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
A.±½·Ö×ÓÖŠŗ¬ÓŠĖ«¼ü£¬ĘäŠŌÖŹøśĻ©ĢžĻąĖĘ
B.±½·Ö×Ó³Ź»·×“½į¹¹£¬ĘäŠŌÖŹÓė»·ĶéĢžĻąĖĘ
C.±½ÓėŅŅČ²¾ßÓŠĻąĶ¬µÄŹµŃéŹ½
D.±½·Ö×ÓĪŖĘ½ĆęÕżĮł±ßŠĪ½į¹¹£¬±½»·ÉĻµÄ6øöĢ¼Ģ¼¼üĶźČ«ĻąĶ¬
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
±¾Ģā°üĄØA”¢BĮ½Š”Ģā£¬·Ö±š¶ŌÓ¦ÓŚ”°ĪļÖŹ½į¹¹ÓėŠŌÖŹ”±ŗĶ”°ŹµŃé»Æѧ”±Į½øöŃ”ŠŽÄ£æéµÄÄŚČŻ£¬ĒėŃ”ŌńĘäÖŠ”ŖĢā£¬²¢ŌŚĻąÓ¦µÄ“šĢāĒųÓņ×÷“š”£ČōĮ½Ģā¶¼×ö£¬Ōņ°“AĢāĘĄ·Ö”£
A£®Ć¾”¢ĶµČ½šŹōĄė×ÓŹĒČĖĢåÄŚ¶ąÖÖĆøµÄøØŅņ×Ó”£¹¤ŅµÉĻ“Óŗ£Ė®ÖŠĢįČ”Ć¾Ź±£¬ĻČÖʱøĪŽĖ®ĀČ»ÆĆ¾£¬Č»ŗó½«ĘäČŪČŚµē½ā£¬µĆµ½½šŹōĆ¾”£
£Ø1£©Ēė²Īæ¼ĻĀŹöŹż¾ŻĢīæÕŗĶ»Ų“šĪŹĢā£ŗ

¹¤ŅµÉĻ³£ÓƵē½āČŪČŚMgCl2µÄ·½·ØÉś²ś½šŹōĆ¾£¬µē½āAl2O3Óė±ł¾§ŹÆČŪČŚ»ģŗĻĪļµÄ·½·ØÉś²śĀĮ”£²»ÓƵē½āMgOµÄ·½·ØÉś²śĆ¾µÄŌŅņ £»²»ÓƵē½āAlCl3µÄ·½·ØÉś²śĀĮµÄŌŅņ ”£
£Ø2£©2001ÄźŌų±ØµĄ£¬ÅšĆ¾»ÆŗĻĪļĖ¢ŠĀĮĖ½šŹō»ÆŗĻĪļ³¬µ¼ĪĀ¶ČµÄ×īøß¼ĒĀ¼”£øĆ»ÆŗĻ¾§Ģå½į¹¹ÖŠµÄ¾§°ūČēĶ¼ĖłŹ¾”£Ć¾Ō×Ó¼äŠĪ³ÉÕżĮłĄāÖł£¬ĮłøöÅšŌ×ÓĪ»ÓŚĄāֳČ”£ŌņøĆ»ÆŗĻĪļµÄ»ÆѧŹ½æɱķŹ¾ĪŖ ”£

£Ø3£©Š“³öCu+µÄŗĖĶāµē×ÓÅŲ¼Ź½ ”£
£Ø4£©ĶłĮņĖįĶČÜŅŗÖŠ¼ÓČė¹żĮæ°±Ė®£¬æÉÉś³É[Cu£ØNH3£©4]2+ÅäĄė×Ó”£ŅŃÖŖNF3ÓėNH3µÄæռ乹ŠĶ¶¼ŹĒČż½Ē׶ŠĪ£¬µ«NF3²»Ņ×ÓėCu2+ŠĪ³ÉÅäĄė×Ó£¬ĘäŌŅņŹĒ ”£
£Ø5£©Ä³ąßöĶŖĄąŅ©Īļ£ØÖŠŠÄĄė×ÓŹĒCu2£«£©½į¹¹ČēĻĀĶ¼£¬¹ŲÓŚøĆŅ©ĪļµÄĖµ·ØÕżČ·µÄŹĒ ”£

A£®ÖŠŠÄĄė×ÓCu2+µÄÅäĪ»ŹżŹĒ5
B£®NŌ×Ó¾ł²ÉÓĆsp2ŌÓ»Æ
C£®“ęŌŚÅäĪ»¼ü”¢¼«ŠŌ¹²¼Ū¼üŗĶ·Ē¼«ŠŌ¹²¼Ū¼ü
D£®ČŪµćŗÜøߣ¬Ó²¶ČŗÜ“ó
B£®Ä³»Æѧъ¾æŠŌѧĻ°Š”×éĪŖĢ½¾æÄ³Ę·ÅĘ»ØÉśÓĶÖŠ²»±„ŗĶÖ¬·¾ĖįµÄŗ¬Į棬½ųŠŠĮĖČēĻĀŹµŃé£ŗ
²½ÖčI£ŗ³ĘČ”0£®4 g»ØÉśÓĶѳʷ£¬ÖĆÓŚĮ½øöøÉŌļµÄµāĘæ£ØČēĶ¼£©ÄŚ£¬¼ÓČė10 mLĖÄĀČ»ÆĢ¼£¬ĒįĒįŅ”¶ÆŹ¹ÓĶČ«²æČܽā”£ĻņµāĘæÖŠ¼ÓČė25£®00 mLŗ¬0£®01 mol IBrµÄĪŽĖ®ŅŅĖįČÜŅŗ£¬øĒŗĆĘæČū£¬ŌŚ²£Į§ČūÓėĘææŚÖ®¼äµĪ¼ÓŹżµĪ10£„µā»Æ¼ŲČÜŅŗ·ā±Õ·ģĻ¶£¬ŅŌĆāIBrµÄ»Ó·¢ĖšŹ§”£

²½ÖčII£ŗŌŚ°µ“¦·ÅÖĆ30 min£¬²¢²»Ź±ĒįĒįŅ”¶Æ”£30 minŗ󣬊”ŠÄµŲ“ņæŖ²£Į§Čū£¬ÓĆŠĀÅäÖʵÄ10£„
µā»Æ¼Ų10 mLŗĶÕōĮóĖ®50 mL°Ń²£Į§ČūŗĶĘæ¾±ÉĻµÄŅŗĢå³åĻ“ČėĘæÄŚ”£
²½Öč¢ó£ŗ¼ÓČėÖøŹ¾¼Į£¬ÓĆ0£®1 mol”¤L£1Įņ“śĮņĖįÄĘČÜŅŗµĪ¶Ø£¬ÓĆĮ¦Õńµ“µāĘ棬ֱÖĮÖÕµć”£
²ā¶Ø¹ż³ĢÖŠ·¢ÉśµÄĻą¹Ų·“Ó¦ČēĻĀ£ŗ
¢Ł
¢ŚIBr£«KI=I2+KBr
¢ŪI2£«2S2O32£=2I££«S4O62£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŅŃÖŖĀ±ĖŲ»„»ÆĪļIBrµÄŠŌÖŹÓėĀ±ĖŲµ„ÖŹĄąĖĘ£¬ŹµŃéÖŠ×¼Č·ĮæČ”IBrČÜŅŗӦєÓƵÄŅĒĘ÷ŹĒ £¬µāĘæ²»øÉŌļ»į·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
£Ø2£©²½Öč¢ņÖŠµāĘæŌŚ°µ“¦·ÅÖĆ30 min£¬²¢²»Ź±ĒįĒįŅ”¶ÆµÄŌŅņŹĒ ”£
£Ø3£©²½Öč¢óÖŠĖł¼ÓÖøŹ¾¼ĮĪŖ £¬µĪ¶ØÖÕµćµÄĻÖĻó ”£
£Ø4£©·“Ó¦½įŹųŗó“ÓŅŗĢå»ģŗĻĪļÖŠ»ŲŹÕĖÄĀČ»ÆĢ¼£¬ĖłŠč²Ł×÷ÓŠ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
£Ø12·Ö£©±¾Ģā°üĄØA”¢BĮ½Š”Ģā£¬·Ö±š¶ŌÓ¦ÓŚ”°ĪļÖŹ½į¹¹ÓėŠŌÖŹ”±ŗĶ”°ŹµŃé»Æѧ”±Į½øöŃ”ŠŽÄ£æéµÄÄŚČŻ£¬ĒėŃ”ŌńĘäÖŠ”ŖĢā£¬²¢ŌŚĻąÓ¦µÄ“šĢāĒųÓņ×÷“š”£ČōĮ½Ģā¶¼×ö£¬Ōņ°“AĢāĘĄ·Ö”£
A£®Ć¾”¢ĶµČ½šŹōĄė×ÓŹĒČĖĢåÄŚ¶ąÖÖĆøµÄøØŅņ×Ó”£¹¤ŅµÉĻ“Óŗ£Ė®ÖŠĢįČ”Ć¾Ź±£¬ĻČÖʱøĪŽĖ®ĀČ»ÆĆ¾£¬Č»ŗó½«ĘäČŪČŚµē½ā£¬µĆµ½½šŹōĆ¾”£
£Ø1£©Ēė²Īæ¼ĻĀŹöŹż¾ŻĢīæÕŗĶ»Ų“šĪŹĢā£ŗ
¹¤ŅµÉĻ³£ÓƵē½āČŪČŚMgCl2µÄ·½·ØÉś²ś½šŹōĆ¾£¬µē½āAl2O3Óė±ł¾§ŹÆČŪČŚ»ģŗĻĪļµÄ·½·ØÉś²śĀĮ”£²»ÓƵē½āMgOµÄ·½·ØÉś²śĆ¾µÄŌŅņ £»²»ÓƵē½āAlCl3µÄ·½·ØÉś²śĀĮµÄŌŅņ ”£
£Ø2£©2001ÄźŌų±ØµĄ£¬ÅšĆ¾»ÆŗĻĪļĖ¢ŠĀĮĖ½šŹō»ÆŗĻĪļ³¬µ¼ĪĀ¶ČµÄ×īøß¼ĒĀ¼”£øĆ»ÆŗĻ¾§Ģå½į¹¹ÖŠµÄ¾§°ūČēĶ¼ĖłŹ¾”£Ć¾Ō×Ó¼äŠĪ³ÉÕżĮłĄāÖł£¬ĮłøöÅšŌ×ÓĪ»ÓŚĄāֳČ”£ŌņøĆ»ÆŗĻĪļµÄ»ÆѧŹ½æɱķŹ¾ĪŖ ”£
£Ø3£©Š“³öCu+µÄŗĖĶāµē×ÓÅŲ¼Ź½ ”£
£Ø4£©ĶłĮņĖįĶČÜŅŗÖŠ¼ÓČė¹żĮæ°±Ė®£¬æÉÉś³É[Cu£ØNH3£©4]2+ÅäĄė×Ó”£ŅŃÖŖNF3ÓėNH3µÄæռ乹ŠĶ¶¼ŹĒČż½Ē׶ŠĪ£¬µ«NF3²»Ņ×ÓėCu2+ŠĪ³ÉÅäĄė×Ó£¬ĘäŌŅņŹĒ ”£
£Ø5£©Ä³ąßöĶŖĄąŅ©Īļ£ØÖŠŠÄĄė×ÓŹĒCu2£«£©½į¹¹ČēĻĀĶ¼£¬¹ŲÓŚøĆŅ©ĪļµÄĖµ·ØÕżČ·µÄŹĒ ”£
A£®ÖŠŠÄĄė×ÓCu2+µÄÅäĪ»ŹżŹĒ5
B£®NŌ×Ó¾ł²ÉÓĆsp2ŌÓ»Æ
C£®“ęŌŚÅäĪ»¼ü”¢¼«ŠŌ¹²¼Ū¼üŗĶ·Ē¼«ŠŌ¹²¼Ū¼ü
D£®ČŪµćŗÜøߣ¬Ó²¶ČŗÜ“ó
B£®Ä³»Æѧъ¾æŠŌѧĻ°Š”×éĪŖĢ½¾æÄ³Ę·ÅĘ»ØÉśÓĶÖŠ²»±„ŗĶÖ¬·¾ĖįµÄŗ¬Į棬½ųŠŠĮĖČēĻĀŹµŃé£ŗ
²½ÖčI£ŗ³ĘČ”0£®4 g»ØÉśÓĶѳʷ£¬ÖĆÓŚĮ½øöøÉŌļµÄµāĘæ£ØČēĶ¼£©ÄŚ£¬¼ÓČė10 mLĖÄĀČ»ÆĢ¼£¬ĒįĒįŅ”¶ÆŹ¹ÓĶČ«²æČܽā”£ĻņµāĘæÖŠ¼ÓČė25£®00 mLŗ¬0£®01 mol IBrµÄĪŽĖ®ŅŅĖįČÜŅŗ£¬øĒŗĆĘæČū£¬ŌŚ²£Į§ČūÓėĘææŚÖ®¼äµĪ¼ÓŹżµĪ10£„µā»Æ¼ŲČÜŅŗ·ā±Õ·ģĻ¶£¬ŅŌĆāIBrµÄ»Ó·¢ĖšŹ§”£
²½ÖčII£ŗŌŚ°µ“¦·ÅÖĆ30 min£¬²¢²»Ź±ĒįĒįŅ”¶Æ”£30 minŗ󣬊”ŠÄµŲ“ņæŖ²£Į§Čū£¬ÓĆŠĀÅäÖʵÄ10£„
µā»Æ¼Ų10 mLŗĶÕōĮóĖ®50 mL°Ń²£Į§ČūŗĶĘæ¾±ÉĻµÄŅŗĢå³åĻ“ČėĘæÄŚ”£
²½Öč¢ó£ŗ¼ÓČėÖøŹ¾¼Į£¬ÓĆ0£®1 mol”¤L£1Įņ“śĮņĖįÄĘČÜŅŗµĪ¶Ø£¬ÓĆĮ¦Õńµ“µāĘ棬ֱÖĮÖÕµć”£
²ā¶Ø¹ż³ĢÖŠ·¢ÉśµÄĻą¹Ų·“Ó¦ČēĻĀ£ŗ
¢Ł
¢ŚIBr£«KI=I2+KBr
¢ŪI2£«2S2O32£=2I££«S4O62£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŅŃÖŖĀ±ĖŲ»„»ÆĪļIBrµÄŠŌÖŹÓėĀ±ĖŲµ„ÖŹĄąĖĘ£¬ŹµŃéÖŠ×¼Č·ĮæČ”IBrČÜŅŗӦєÓƵÄŅĒĘ÷ŹĒ £¬µāĘæ²»øÉŌļ»į·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
£Ø2£©²½Öč¢ņÖŠµāĘæŌŚ°µ“¦·ÅÖĆ30 min£¬²¢²»Ź±ĒįĒįŅ”¶ÆµÄŌŅņŹĒ ”£
£Ø3£©²½Öč¢óÖŠĖł¼ÓÖøŹ¾¼ĮĪŖ £¬µĪ¶ØÖÕµćµÄĻÖĻó ”£
£Ø4£©·“Ó¦½įŹųŗó“ÓŅŗĢå»ģŗĻĪļÖŠ»ŲŹÕĖÄĀČ»ÆĢ¼£¬ĖłŠč²Ł×÷ÓŠ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com