
��1��д����ҵ�Ϸֱ��Ԣ�CH2��CH2Ϊԭ�Ϻ͢�C6H12O6Ϊԭ����ȡ�Ҵ���
��ѧ����ʽ���� ��
��2����֪���ӷ�Ӧ����H2A��B����HA����HB ��H2A��2C����A2����2HC
��H2A��HA����HB��HC��������ǿ����������˳��Ϊ
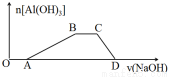
��3�����е����ʵ���Ũ�ȵ�HCl��NH4Cl��AlCl3�����Һ�еμ�NaOH��Һ�����ɳ��������ʵ��������NaOH��Һ������仯��ϵ��ͼ����д����OA�κ͢�CD�η�����Ӧ�����ӷ���ʽ���� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017������ʡ��«������УЭ����������ڳ����Ի�ѧ�Ծ��������棩 ���ͣ������
�״��ǽṹ��Ϊ�ı���һԪ�����ֳơ�ľ������ľ�������״���һ̼��ѧ������ԭ�Ϻ����ʵ�ȼ�ϣ���ҪӦ���ھ�ϸ���������ϡ���Դ��������֪�״��Ʊ����йػ�ѧ��Ӧ���£�
��Ӧ�٣�CO(g)��2H2(g)  CH3OH(g) ��H1����90.77kJ/mol
CH3OH(g) ��H1����90.77kJ/mol
��Ӧ�ڣ�CO2(g)��H2(g)  CO(g)��H2O(g) ��H2
CO(g)��H2O(g) ��H2
��Ӧ�ۣ�CO2(g)��3H2(g)  CH3OH(g)��H2O(g) ��H3����49.58kJ/mol
CH3OH(g)��H2O(g) ��H3����49.58kJ/mol
��1����Ӧ�ڵġ�H2��__________________
��2����500��ʱ������Ӧ��ƽ�ⳣ������ΪK1��K2��K3����֪500��ʱK1��K2��ֵ�ֱ�Ϊ2.5��1.0������ø��¶��·�Ӧ����ijʱ�̣�H2(g)��CO2(g)��CH3OH(g)��H2O(g)��Ũ��(mol/L)�ֱ�Ϊ0.8��0.1��0.3��0.15�����ʱV��________V��(�����������������)
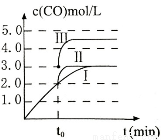
��3����3L�ݻ��ɱ���ܱ������з�����Ӧ�ڣ�c(CO)�淴Ӧʱ��t�仯��ͼ������I��ʾ������t0ʱ�̷ֱ�ı�һ������������I��Ϊ����II������III��������I��Ϊ����IIʱ���ı�������� ����ͨ���ı�ѹǿʹ����I��Ϊ����IIIʱ������III�ﵽƽ��ʱ���������Ϊ_____________��
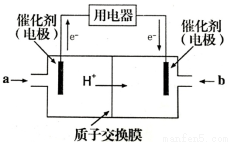
��4���״�ȼ�ϵ�ؿ��ܳ�Ϊδ����Я���Ӳ�ƷӦ�õ�������ij�ּ״�ȼ�ϵ�ع���ԭ����ͼ��ʾ����ͨ��a����ĵ缫�缫��ӦʽΪ ��
��5��һ�������¼״���һ����̼��Ӧ���Ժϳ����ᡣ���������£���a mol/L��CH3COOH��b mol/LBa(OH)2��Һ�������ϣ���Ӧƽ��ʱ��2c(Ba2��)��c(CH3COO��)���ú�a��b�Ĵ���ʽ��ʾ�û����Һ�д���ĵ��볣��Ϊ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ������ٿ�ѧ���л�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����й�����������ۼ�������ӷ���ʽ����ȷ���ǣ� ��
ѡ�� | ������ | ���ۼ����ӷ���ʽ |
A | H+��Fe2+��NO3-��Cl- | ���ܴ���������ͬһ��Һ��,��Ϊ������Ӧ:Fe2++2H+=Fe3++ H2�� |
B | Na+��K+��HCO3-��OH- | ���ܴ���������ͬһ��Һ��,��Ϊ������Ӧ: HCO3-+ OH-=H2O+CO2�� |
C | Ca2+��NH4+��CO32-��Cl- | �ܴ���������ͬһ��Һ�� |
D | Na+��NH4+��SO42-��Cl- | �ܴ���������ͬһ��Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ������ѧ���ڳ��������Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
X��Y��Z��W��ԭ��������������Ķ�����Ԫ�ء�X��Wͬ���壬Z��Wͬ���ڣ�Xԭ�Ӻ�����������Yԭ�Ӻ�����������3/4��Wԭ��������������Zԭ��������������4��������˵������ȷ���ǣ� ��
A. ԭ�Ӱ뾶��r(X)��r(Y)��r(W)��r(Z)
B. Ԫ��W����̬�⻯������ȶ��Ա�Ԫ��X��ǿ
C. X��Y��Z����Ԫ���γɵĻ������д������Ӽ����ۼ�
D. Y��W�γɵĻ�������۵�϶���Y��Z�γɵĻ�������۵��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ������ѧ���ڳ��������Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��ѧ������������������ء����й�����û�з�����ѧ�仯���ǣ� ��
A��������ˮɱ�������� B���轺����װʳƷ�ĸ����
C������������ֽ����Ư�� D������ˮ���ó涣ҧ������ϴ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����и�����ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ӷ���ʽ�У�ֻ�ܱ�ʾһ����ѧ��Ӧ����( )
��Ag����Cl��===AgCl��
��Ba2����2OH����2H����SO42��===BaSO4����2H2O
��CO32����2H��===CO2����H2O
��Fe��Cu2��===Fe2����Cu
A���٢� B���ڢ� C���ڢ� D��û��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����и�����ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵������ȷ����( )
A�������ǻ����л����Ϊ��
B���ܷ���������Ӧ���л��ﶼ��ȩ
C�������׳�ʯ̿�ᣬ���Ա�̼��ǿ
D����������֬����ɷֵĸ�����һ��ʹ�̬
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016��ɽ��ʡҵˮƽ��ѧģ���Ծ���4���������棩 ���ͣ�ѡ����
���淴Ӧ��һ�������´ﵽ��ѧƽ��״̬�ı�־�ǣ� ��
A����Ӧֹͣ��
B������Ӧ�������淴Ӧ������Ⱦ�Ϊ��
C����Ӧ���������Ũ�����
D����Ӧ���������Ũ�Ȳ��ٱ仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�긣��ʡȪ����ѧҵˮƽ��ѧģ���Ծ����ģ��������棩 ���ͣ�ѡ����
�������ʻ�Ϊͬ���칹����ǣ� ��
A��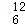 C��
C��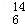 C B�������ͳ���
C B�������ͳ���
C����������� D����������춡��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com