
�л��������ұ�(GB2760��200��)�涨���Ѿ���SO2���ʹ����Ϊ0.25g��L��1.ij��ȤС������9ͼIװ��(�г�װ����)�ռ�ij���Ѿ���SO2,�����京�����вⶨ.
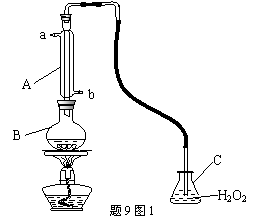

 (1)����A�������� ��ˮͨ��A�Ľ���Ϊ ��
(1)����A�������� ��ˮͨ��A�Ľ���Ϊ ��
(2)B�м���300.00mL���Ѿƺ��������ᣬ����ʹSO2ȫ���ݳ�����C��H2O2��ȫ��Ӧ���仯ѧ����ʽΪ ��
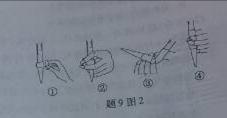
(3)��ȥC�й�����H2O2��Ȼ����0.0900mol��L��1NaOH����Һ���еζ����ζ�ǰ������ʱ��Ӧѡ����9ͼ2�еģ����ζ��յ�ʱ��Һ��pH��8.8����ѡ���ָʾ��Ϊ������50mL�ζ��ܽ���ʵ�飬���ζ����е�Һ���ڿ̶ȡ�10�����������Һ������ (�����)(�٣�10mL���ڣ�40mL���ۣ�10mL���ܣ�40mL)
(4)�ζ����յ�ʱ������NaOH��Һ25.00mL�������Ѿ���SO2����Ϊ g��L��1
(5)�òⶨ�����ʵ��ֵƫ�ߣ�����ԭ����������װ������Ľ���ʩ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
20��ʱ��NaCl�ܽ���ˮ��ʵ���������±���
| ʵ����� | ����ˮ������/g | NaCl����/g | δ�ܵ�NaCl����/g |
| �� | 10 | 4 | 2 |
| �� | 20 | 4 | 0 |
| �� | 30 | 4 | 0 |
A��ʵ���������ҺΪ������Һ B��ʵ���������ҺΪ������Һ
C��20��ʱNaCl���ܽ��Ϊ2g D��ʵ���������Һ��������������Ϊ13.3%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������������Ե�10�ǻ�ϲ����Ľṹ��ͼ��ʾ��
���й���10�ǻ�ϲ�����˵����ȷ����
A. ����ʽΪC20H16N2O5
B. ������FeCl3��Һ������ɫ��Ӧ
C. ���ܷ���������Ӧ
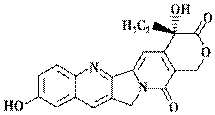 D. һ�������£�1 mol������������1 mol NaOH��Ӧ
D. һ�������£�1 mol������������1 mol NaOH��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Na��Cu��O��Si��S��Cl�dz���������Ԫ�ء�
��1��Naλ��Ԫ�����ڱ��� ���ڵ� �壻S�Ļ�̬ԭ�Ӻ����� ��δ�ɶԵ��ӣ�Si�Ļ�̬ԭ�Ӻ�������Ų�ʽΪ ��
��2���á�����������գ�
| ��һ������ | ���Ӱ뾶 | �۵� | ���� |
| Si S | O2�� Na�� | NaCl Si | H2SO4 HClO4 |
��3��CuCl(s)��O2��Ӧ����CuCl2(s)��һ�ֺ�ɫ���塣��25 �桢101KPa�£���֪�÷�Ӧÿ����1 mol CuCl2(s)������44.4KJ���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��4��ClO2�dz�����ˮ�ľ�������ҵ�Ͽ���Cl2����NaClO2��Һ��ȡClO2��д���÷�Ӧ�����ӷ���ʽ�����������ת�Ƶķ������Ŀ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ҷ����Ԫ�صļ���ɾ��������ĸ��������,��������ѡ�õ�ʵ����Ʒ���ܶ��õ�����( )


A������Ҷ���ջһ���ѡ�â١��ں͢�
B����Ũ�����ܽ��Ҷ�Ҳ�������ˮϡ�ͣ�ѡ�âܡ��͢�
C�����˵õ�����Һ��ѡ�âܡ��ݺ͢�
D��������Һ�е�Fe3����ѡ�âۡ���͢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�ֽ�1molH2O2�ų�����98kJ���ں�����I—����Һ�У�H2O2�ֽ�Ļ���Ϊ��
 H2O2+I��
H2O2+I�� H2O+IO�� ���� H2O2+IO��
H2O+IO�� ���� H2O2+IO�� H2O+O2+I�� ��
H2O+O2+I�� ��
A����Ӧ������I—Ũ���й� B��IO—Ҳ�Ǹ÷�Ӧ�Ĵ���
C����Ӧ��ܵ���98kJ/mol D��v(H2O2)=v(H2O)=v(O2)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ҵ�����Ҫ���л�����ԭ�ϣ�������ϩ����ֱ��ˮ�Ϸ����ˮ�Ϸ��������ش��������⣺
��1�����ˮ�Ϸ���ָ�Ƚ���ϩ��Ũ���ᷴӦ����������������C2H5OSO3H������ˮ�������Ҵ���д����Ӧ��Ӧ�Ļ�ѧ����ʽ__________________________________��
��2����֪��
�״���ˮ��Ӧ 2CH3OH(g)=CH3OCH3(g)+H2O(g) ��H=-23.9kJ/mol
�״���ϩ����Ӧ 2CH3OH(g)=C2H4(g)+2H2O(g) ��H=-29.1kJ/mol
�Ҵ��칹����Ӧ C2H5OH(g)=CH3OCH3(g) ��H=+50.7kJ/mol
����ϩ����ֱ��ˮ�Ϸ�ӦC2H4(g)+2H2O(g)= C2H5OH(g)�ġ�H=________kJ/mol������ˮ�Ϸ���ȣ�����ֱ��ˮ�Ϸ����ŵ���___________________________��
��3����ͼΪ����ˮ�Ϸ�����ϩ��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��n(H2O):n(C2H4)=1:1����
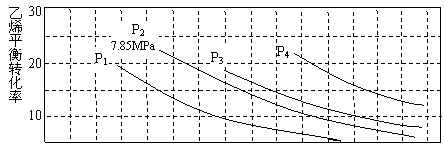 |
|
������ʽ������ϩˮ�����Ҵ���Ӧ��ͼ��A���ƽ�ⳣ��Kp=_______________
_____________������ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ=��ѹ�����ʵ�����������
��ͼ��ѹǿ��P1��P2��P3��P4���Ĵ�С˳��Ϊ___________��������_________________��
������ֱ��ˮ�Ϸ������õĹ�������Ϊ������/������Ϊ��������Ӧ�¶�290�桢
ѹǿ6.9MPa��n(H2O):n(C2H4)=0.6:1����ϩ��ת����Ϊ5%����Ҫ��һ�������ϩת���ʣ����˿����ʵ��ı��¶Ⱥ�ѹǿ�⣬�����Բ�ȡ�Ĵ�ʩ��___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������������ڴ������һ���� (����)
�ٱ�ˮ�����ڱ������������ȼ�������ͨ��������ˮ��������Ư�ۡ��߶��ױ�����TNT�����屽����C5H10 ⑪����40%������þ��⑫�����͡�⑬�������֡�⑭�ܷⱣ���NO2���塡⑮CuSO4��5H2O��⑯KAl(SO4)2��12H2O ⑰������ϩ��⑱Һ��
A���ڢۢܢ� B���ߢ�⑫⑬⑭⑮⑯
C���٢��⑪⑮⑯⑱ D���٢ܢ��⑪⑫⑰⑱
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ж�������ȷ�Ļ����̡�������Ļ�������
(1)ǿ�������Һ�ĵ����Ա����������Һ�ĵ�����ǿ (����)
(2)BaSO4Ͷ��ˮ�У� �����Խ������������������ (����)
(3)���������Һ�д������ֹ��ۻ�������� (����)
(4)ǿ����ʶ������ӻ����������ʶ��ǹ��ۻ����� (����)
(5)CaO��ǿ����ʣ�����Ϊ����ˮ��Һ�ܵ��� (����)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com