
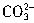 ��H����
��H����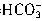 ��Ӧ���յ㣩������HCl��Һ���ΪV1mL���ټ�1��2�μ���ָʾ����������HCl��Һ�ζ���Һ�ɻ�ɫ���ɫ������HCl��Һ�����ΪV2mL��д��������Ʒ��NaHCO3���������ļ���ʽ���أ�NaHCO3����_______��
��Ӧ���յ㣩������HCl��Һ���ΪV1mL���ټ�1��2�μ���ָʾ����������HCl��Һ�ζ���Һ�ɻ�ɫ���ɫ������HCl��Һ�����ΪV2mL��д��������Ʒ��NaHCO3���������ļ���ʽ���أ�NaHCO3����_______�� 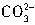 +H+=
+H+=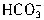 ����
����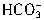 +H+=H2O+CO2������Ӧ��������n��H+��=cV1��10-3mol����Щ
+H+=H2O+CO2������Ӧ��������n��H+��=cV1��10-3mol����Щ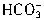 ת��ΪCO2ͬ����ȥn��H+��ΪcV1��10-3mol���ʽ�
ת��ΪCO2ͬ����ȥn��H+��ΪcV1��10-3mol���ʽ�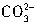 ת��ΪCO2����ȥn��H+����=2cV1��10-3mol��������е�NaHCO3��
ת��ΪCO2����ȥn��H+����=2cV1��10-3mol��������е�NaHCO3��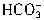 ��ת��ΪCO2��ȥn��H+��=c��V2-2V1����10-3mol����n��NaHCO3��=c��V2-2V1����10-3mol������Ʒ�У��أ�NaHCO3����
��ת��ΪCO2��ȥn��H+��=c��V2-2V1����10-3mol����n��NaHCO3��=c��V2-2V1����10-3mol������Ʒ�У��أ�NaHCO3����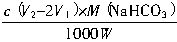 ��100%��
��100%�� 

 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 |
0�� | 10�� | 20�� | 30�� | 40�� | 50�� | 60�� | 100�� |
| NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | 39.8 |
| NH4HCO3 | 11.9 | 15.8 | 21.0 | 27.0 | -�� | |||
| NaHCO3 | 6.9 | 8.1 | 9.6 | 11.1 | 12.7 | 14.5 | 16.4 | |
| NH4Cl | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.3 | 77.3 |
| c(v2-v1)106 |
| 1000w |
| c(v2-v1)106 |
| 1000w |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �¶��ܽ���� | 0�� | 10�� | 20�� | 30�� | 40�� | 50�� | 60�� | 100�� |
| NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | 39.8 |
| NH4HCO3 | 11.9 | 15.8 | 21.0 | 27.0 | -�� | - | - | - |
| NaHCO3 | 6.9 | 8.1 | 9.6 | 11.1 | 12.7 | 14.5 | 16.4 | - |
| NH4Cl | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.3 | 77.3 |
| c(V2-V1)M |
| 1000W |
| c(V2-V1)M |
| 1000W |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
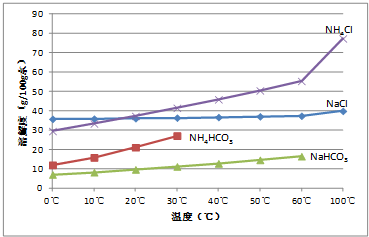
�������ڲ�ͬ�¶��µ��ܽ�ȣ�g/100 gˮ����
�¶� �ܽ�� �� | 0 �� | 10 �� | 20 �� | 30 �� | 40 �� | 50 �� | 60 �� | 100 �� |
NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | 39.8 |
NH4HCO3 | 11.9 | 15.8 | 21.0 | 27.0 | ���� | �� | �� | �� |
NaHCO3 | 6.9 | 8.1 | 9.6 | 11.1 | 12.7 | 14.5 | 16.4 | �� |
NH4C | l29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.3 | 77.3 |
�٣�35�� NH4HCO3���зֽ�
��ش�
��1����Ӧ�¶ȿ�����30��35 �棬����Ϊ������35 �棬��__________________________��������30 �棬��______________________________________��Ϊ���ƴ��¶ȷ�Χ����ȡ�ļ��ȷ���Ϊ__________________________________________________________��
��2��������Ϻ�������30���ӣ�Ŀ����______________________________________�����ú�ֻ����NaHCO3�����ԭ����___________________________________________��������ˮϴ��NaHCO3�����Ŀ���dz�ȥ_______________________________________���ʣ��Ի�ѧʽ��ʾ����
��3���������õ�ĸҺ�к���______________________________________���Ի�ѧʽ��ʾ���������___________________��������һ��������ʹNaCl��Һѭ��ʹ�ã�ͬʱ�ɻ���NH4Cl��
��4�����Դ����Ʒ��NaHCO3�����ķ����ǣ�ȷ��ȡ������ƷW g��������ƿ�м�����ˮ�ܽ⣬��1��2�η�ָ̪ʾ���������ʵ���Ũ��Ϊc( mol��L-1)��HCl��Һ�ζ�����Һ�ɺ�ɫ����ɫ��ָʾ![]() +H+====
+H+====![]() ��Ӧ���յ㣩������HCl��Һ���ΪV1 mL���ټ�1��2�μ���ָʾ����������HCl��Һ�ζ�����Һ�ɻƱ�ȣ�����HCl��Һ�����ΪV2 mL��д��������Ʒ��NaHCO3���������ļ���ʽ��
��Ӧ���յ㣩������HCl��Һ���ΪV1 mL���ټ�1��2�μ���ָʾ����������HCl��Һ�ζ�����Һ�ɻƱ�ȣ�����HCl��Һ�����ΪV2 mL��д��������Ʒ��NaHCO3���������ļ���ʽ��
w(NaHCO3)=___________________��
?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�������ڲ�ͬ�¶��µ��ܽ��(g/
�¶� �ܽ�� �� | ||||||||
NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | 39.8 |
NH4HCO3 | 11.9 | 15.8 | 21.0 | 27.0 | ���� | �� | �� | �� |
NaHCO3 | 6.9 | 8.1 | 9.6 | 11.1 | 12.7 | 14.5 | 16.4 | �� |
NH4Cl | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.3 | 77.3 |
(˵�����٣�
��ش��������⣺
(1)װ�õ�����˳��Ӧ��__________(����ĸ)��
(2)Aװ����ʢ�ŵ��Լ���__________����������__________��
(3)��ʵ������У���Ҫ����D�¶���30��
(4)��Ӧ��������ƿ������ˮ������NaHCO3�����ԭ����____________________
_____________________��������ˮϴ��NaHCO3�����Ŀ���dz�ȥ____________����(�Ի�ѧʽ��ʾ)��
(5)���Դ����Ʒ��NaHCO3�����ķ����ǣ�ȷ��ȡ������ƷW g��������ƿ�м�����ˮ�ܽ⣬��1��2�η�ָ̪ʾ���������ʵ���Ũ��Ϊc mol��L-1��HCl��Һ�ζ�����Һ�ɺ�ɫ����ɫ(ָʾ![]() ��H��
��H��![]()
![]() ��Ӧ���յ�)������HCl��Һ���ΪV1 mL���ټ�1��2�μ���ָʾ����������HCl��Һ�ζ���Һ�ɻƱ�ȣ�����HCl��Һ���ΪV2 mL��д��������Ʒ��NaHCO3���������ļ���ʽ��NaHCO3(%)��_______________________________��
��Ӧ���յ�)������HCl��Һ���ΪV1 mL���ټ�1��2�μ���ָʾ����������HCl��Һ�ζ���Һ�ɻƱ�ȣ�����HCl��Һ���ΪV2 mL��д��������Ʒ��NaHCO3���������ļ���ʽ��NaHCO3(%)��_______________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com