
���� ��1����������һ�����ʵ���Ũ����Һ��һ�㲽����
��2���������Ʋ����Ǽ��㡢�������ܽ⡢ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������Ҫ��������
��3������m=CVM������Ҫ���ʵ�������
��4�����ݵ���ȷ��������ʼֱ��������ƿ�м�������ˮ����Һ��ඨ�ݿ̶���1��2���״������õιܵμӣ�ʹ��Һ����Ͷ���̶������У�
��5�������������������ʵ����ʵ�������Һ�������Ӱ�죬����C=$\frac{n}{V}$������������
��� �⣺��1������һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȣ�
�ʴ�Ϊ���������ܽ⡢��Һ�����ݣ�
��2��0.1mol/LNaOH��Һ450ml��Ӧѡ��500ml����ƿ�����������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ�����ձ��������ò��������裬�����ܽ⣮��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ���������������������ƽ���ձ�����������500mL����ƿ����ͷ�ιܣ����Ի���Ҫ���У���������500ml����ƿ����ͷ�ιܣ�
�ʴ�Ϊ����������500ml����ƿ����ͷ�ιܣ�
��3����Ҫ0.1mol/LNaOH��Һ450ml��Ӧѡ��500ml����ƿ������500ml��Һ����Ҫ�������Ƶ�����=0.1mol/L��0.5L��40g��mol=2.0g��
�ʴ�Ϊ��2.0g��
��4�����ݵ���ȷ��������ʼֱ��������ƿ�м�������ˮ����Һ��ඨ�ݿ̶���1��2���״������õιܵμӣ�ʹ��Һ����Ͷ���̶������У�
�ʴ�Ϊ����ʼֱ��������ƿ�м�������ˮ����Һ��ඨ�ݿ̶���1��2���״������õιܵμӣ�ʹ��Һ����Ͷ���̶������У�
��5��A����NaOH����ֽ���ϳ����������������ƾ�����ʪ�ԣ����ʵ�����ƫС�����ʵ���ҲƫС��������Һ��Ũ��ƫС��
B��������ƿת��ʱ��������Һ�彦�������ʵ�����ƫС�����ʵ���ҲƫС��������Һ��Ũ��ƫС��
C��δ��ȴ������ת�Ƶ�����ƿ�У�������Һ�����ƫС��������Һ��Ũ��ƫ��
D������ʱ�����ӿ̶��ߣ�������Һ�����ƫ��������Һ��Ũ��ƫС��
E������ƿδ���T����������Һ�������ʵ����ʵ�������Һ��������������Ӱ�죬��Һ��Ũ�Ȳ��䣻
�ʴ�Ϊ��C��AB��E��
���� ���⿼��һ�����ʵ���Ũ����Һ�����Ƽ�����������ȷ����ԭ�����̣�ע��ʵ��������ѡ�������жϷ������ɽ����Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
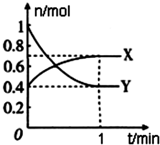 ��һ���¶��£����Ϊ4L���ܱ������У�NO2��N2O4֮�䷢����Ӧ��2NO2��g��������ɫ��?N2O4��g������ɫ������ͼ��ʾ��
��һ���¶��£����Ϊ4L���ܱ������У�NO2��N2O4֮�䷢����Ӧ��2NO2��g��������ɫ��?N2O4��g������ɫ������ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ֲ���ͺ��Ҵ� | B�� | ����ˮ | C�� | �ƾ���ˮ | D�� | ���ͺ�ú�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʯ�ͳ�ѹ����ɵ�ֱ�����ͣ�ʯ����������ʯ���ѻ���ԭ�� | |
| B�� | ʯ���ѽ��Ŀ����Ϊ�˵õ���ϩ����ϩ�ͼ���Ȼ���ԭ�� | |
| C�� | ú�ĸ������������仯��ʯ�͵ļ�ѹ�����ǻ�ѧ�仯 | |
| D�� | ú�к��б��ͼױ�������ͨ���ȸ�������ķ����õ����ͼױ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com