
��ˮ�Ƿḻ����Դ���⣬�Ӻ�ˮ����ȡԪ���ǻ�ѧ��ҵ����Ҫ��ɲ��֡�
��1�����ξ��ƾ��dz�ȥ���е�Ca2+��Fe3+��SO42-����ɳ�����ʣ��������Լ��У���Na2CO3��Һ ��HCl�����ᣩ ��Ba��OH��2��Һ���������Լ�������˳����_________������ţ���
��2�����������С���ˮ���塱����ȡ�嵥�ʷ�Ӧ�����ӷ���ʽΪ��__________��
��3��ijͬѧ�������ͼװ�ý������µ绯ѧʵ�顣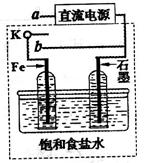
�ٵ�����K��a����ʱ�������������ݲ�����������Ϊ_______����
��һ��ʱ���ʹ����K��a�Ͽ�����b����ʱ�����߿��ڵ�װ�ÿɳ�Ϊ__________����д����ʱFe�缫�ϵĵ缫��Ӧʽ_________________��
��4��ij������ʢ��CaSO4����Һ�ķ�Ӧ����ͨ�백������ȡ���ʣ�NH4��2SO4��Ч�����á���ͨ��CO2��������������NH4��2SO4���������ԭ�� ��
��
��1���ۢ٢ڣ�2�֣�
��2��Cl2��2Br��=2Cl����Br2��2�֣�
��3����Fe����������2�֣�
��ԭ��أ�2�֣���Fe��2e����2OH��= Fe(OH)2����3�֣�
��4��CO2���ڰ�ˮ��������CO32-��CO2+2NH3��H2O =��NH4��2CO3+ H2O��1�֣� ��CO32-������Ʒ�Ӧ�����ܶȻ�������С��̼��Ƴ�����CaSO4��s����CO32-��aq��= CaCO3��s����SO42-��aq����2�֣���ʹ����Ƶij����ܽ�ƽ�����ܽ�ķ����ƶ���3�֣����Ӷ�����������NH4�� 2SO4������3�֣�
���������������1��Na2CO3�ȳ�ȥCa2+������ȥ������Ba��OH��2��������Na2CO3���������������˳��Ϊ���ۢ٢ڡ�
��3������Ϊ���缫�������ݲ�����Fe�缫ֻ����������
�ڿ���K��a�Ͽ�����b����ʱ���γ�ԭ��ء�
��4���������Ա�Ksp���Կ���CaCO3�����ܣ�����ͨ��CO2�백ˮ���ɣ�NH4��2CO3��CO32?ʹCaSO4ת��Ϊ��NH4�� 2SO4��
���㣺 ���⿼���˳����Լ���ѡ�����ӷ���ʽ����д���绯ѧ�ͳ����ܽ�ƽ�⡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ͼװ����ʾ��C��D��E��F���Ƕ��Ե缫���ס�������Һ�������Ũ�ȶ���ͬ(����ͨ��ǰ����Һ�������)��A��BΪ���ֱ����Դ����������ֱ����Դ��ͨ��F�������ʺ�ɫ����ش�
��1�� һ��ʱ�������Һ��ɫ ��
д������C�ĵ缫��Ӧʽ ��
��2�� ���ס���װ���е�C��D��E��F�缫��ֻ��һ�ֵ�������ʱ����Ӧ���ʵ����ʵ���֮��Ϊ ��
��3�� ���ñ�װ�ø�ͭ�����������Һ�� ��Һ����������Һ��pH��13ʱ(��ʱ����Һ���Ϊ500 mL)�����жƼ���������������Ϊ ��
��4�� ����F�缫����Ϊ��������װ�ö����䣬�����з����ܷ�Ӧ�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ͼ��ʾ��װ���У���ͨ��ֱ����5 minʱ��ͭ�缫��������2.16 g���Իش�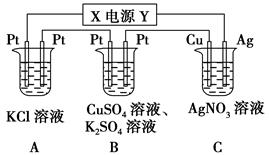
��1����Դ�缫X������Ϊ________��
��2��pH�仯��A________��B________��C________��(�������С�����䡱)
��3��ͨ��5 min��B�й��ռ�224 mL����(��״��)����Һ���Ϊ200 mL����ͨ��ǰCuSO4��Һ�����ʵ���Ũ��Ϊ________(����ǰ����Һ����ޱ仯)��
��4����A��KCl��Һ�����Ҳ��200 mL��������Һ��OH�������ʵ���Ũ��Ϊ________(����ǰ����Һ����ޱ仯)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾװ���У��ס��ҡ��������ձ����ηֱ�ʢ��109g5.51����NaOH��Һ��������CuSO4��Һ��200g10.00����K2SO4��Һ���缫��Ϊʯī�缫��
��ͨ��Դ������һ��ʱ���ñ���K2SO4Ũ��Ϊ10.47��������c�缫�������ӡ��ݴ˻ش����⣺
��1���缫b�Ϸ����ĵ缫��ӦΪ___________________________________��
��2���缫b�����ɵ������ڱ�״���µ����Ϊ__________________����ʱ���ձ���NaOH��Һ�����ʵ���Ũ��Ϊ������Һ���ܶ�Ϊ1g/cm3��_______________��
��3���缫c�������仯��___________g����ʹ�������еĵ��Һ�ָ�����ʼ״̬��Ӧ������Һ�м���������___________������ĸ��ţ���
| A��Cu(OH)2 | B��Cu2O | C��CuCO3 | D��Cu2(OH)2CO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��η�ֹ������ʴ�ǹ�ҵ���о����ص����ݡ�Ϊ�о�������ʴ������ͼ��ʾ�������ڢ١��ڡ������ֲ�ͬ�Ļ����С���ش�
��1��������ʴ��Ҫ��Ϊ ��ʴ�� ��ʴ���֡�
��2��������ʴ�������ɿ쵽����˳���� ������ţ���
��3�����������ĵ缫��ӦʽΪ ��
�����ĵ缫��ӦʽΪ ��
���и����ĵ缫��ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����Ч�ļ���ȼ�ϵ�ز��ò�Ϊ�缫���ϣ����缫�Ϸֱ�ͨ��CH4��O2�������ΪKOH��Һ��ij�о�С�齫����ȼ�ϵ����Ϊ��Դ�����Ȼ�þ��Һ���ʵ�飬���װ����ͼ��ʾ��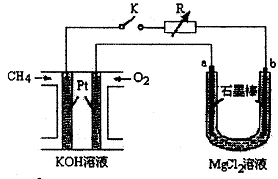
��ش��������⣺
��1������ȼ�ϵ�ظ����ĵ缫��ӦʽΪ�� ��
��2���պϿ���K��a��b�缫�Ͼ����������������a�缫�ϵ�������� ���飬b�缫�ϵõ��������� ������Ȼ�þ��Һ�����ӷ���ʽΪ ��
��3��������ͨ����Ϊ1.12 L����״�������ҷ�Ӧ��ȫ����������ͨ�����صĵ��ӵ����ʵ���Ϊ ���������������Ϊ L����״������
��4����֪���³�ѹ�£�0.25molCH4��ȫȼ������CO2��H2Oʱ���ų�222.5kJ��������д��CH4ȼ���ȵ��Ȼ�ѧ����ʽ ��
��֪����C��ʯī��+O2��g��=CO2��g����H1=-393��5kJ/mol
��2H2��g��+O2��g��=2H2O��l����H2=-571��6kJ/mol
���㣺C��ʯī����H2��g����Ӧ����1molCH4��g���ġ�H= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ͼ��ʾ��ijͬѧ�����һ��ȼ�ϵ�ز�̽���ȼҵԭ���ʹ�ͭ�ľ���ԭ����������װ����XΪ�����ӽ���Ĥ���밴Ҫ��ش��������:
��1������ȼ�ϵ�ظ����缫��Ӧʽ��:
��2��ʯī�缫(C)�ĵ缫��ӦʽΪ
��3�����ڱ�״���£���2�� 24 L�����μӷ�Ӧ������װ�������缫�����ɵ��������Ϊ_ L;��װ������������ͭ������Ϊ g
��4��ijͬѧ������ȼ�ϵ����Ƶ�ⷨ��ȡƯ��Һ��Fe(OH)2��ʵ��װ��(��ͼ��ʾ)����������Ư��ҺʱaΪ���_ �����������Һ�����_ ���������� Fe(OH)2��ʹ�����������������Һ������ѡ�� ���缫��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�á���ͼ��ʾһ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ������
��ش��������⣺
��1����X��Y���Ƕ��Ե缫��a�DZ���NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ����
�ٵ�����X���ϵĵ缫��ӦʽΪ ��
��X�������۲쵽�������� ����
��Y�缫�ϵĵ缫��ӦʽΪ ��
����õ缫��Ӧ����ķ����� ��
��2����Ҫ�õ�ⷽ��������ͭ�����Һaѡ��CuSO4��Һ����
��X�缫�IJ����� ���缫��Ӧʽ�� ��
��Y�缫�IJ����� ���缫��Ӧʽ�� ��
��˵�������ʷ����ĵ缫��Ӧ����д����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
(16��)ij�о�С�����ô�ʳ��(��Ca2+��Mg2+��SO ��)���ֹ�(��C������Cl2��Ӧ�Ĺ�������)��ȡ���裬������µĹ������̣�
��)���ֹ�(��C������Cl2��Ӧ�Ĺ�������)��ȡ���裬������µĹ������̣�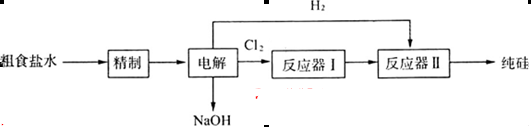
�Իش��������⣺
��1����ҵ��һ�������ù�����̿�ڸ����»�ԭʯӢɰ����ȡ�ֹ裬д���ù��̵Ļ�ѧ����ʽ��_______________________________________________________________________��
��2�����ƴ���ˮ�����Լ�Ϊ��BaC12����Na2CO3����HC1����NaOH����μӵ��Ⱥ�˳�������е�________(�����и�������)��
a���٢ڢܢ� b���ܢڢ٢� c���ܢ٢ۢ� d���ڢܢ٢�
��֪�� ������ô���ˮ��
������ô���ˮ�� ��Ũ�Ⱦ�Ϊ0.01 mol��L-1������1 L�ô���ˮ������һ����Na2CO3��Һ�����ȳ��ֵij�����__________��
��Ũ�Ⱦ�Ϊ0.01 mol��L-1������1 L�ô���ˮ������һ����Na2CO3��Һ�����ȳ��ֵij�����__________��
��3����֪SiCl4�ķе���57.6�棬CC14�ķе���76.8�档�ڷ�Ӧ��I�еõ���SiCl4��Ʒ�к���CCl4�����еõ�����SiCl4�ɲ��õķ��������и����е�________(�����)��
a������ b������ c����Һ d������
��Ӧ�����з�����Ӧ�Ļ�ѧ����ʽΪ__________________________________________��
��4����ͼ�������ӽ���Ĥ����ⱥ��ʳ��ˮ��ʾ��ͼ������������������������_____����ƷA�Ļ�ѧʽΪ____________��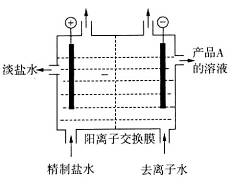
��������Ĥ���۵�ⱥ��ʳ��ˮ����ȡ�������ƣ���д���÷�Ӧ�Ļ�ѧ����ʽ__ ___��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com