
(8��)ʵ������ NaHSO4 ,Ba(OH)2, NH3 ? H2O NaHCO3��KAl(SO4)2������ɫ��Һ������ͨ������֮������Ӧ���������м��𡣲������ʼ�ķ�Ӧ�������±���
���С� ����ʾ�����������ʣ���
����ʾ�����������ʣ��� ����ʾ���ɳ�����
����ʾ���ɳ�����
����������Ϣ���ش��������⣺
(1)B��E�Ļ�ѧʽ�ֱ�Ϊ_______��___________��
(2) д��A�ĵ��뷽��ʽ___________________________����
(3) C��D����Һ��Ӧ�����ӷ���ʽΪ___________________________��
(4) ����0.1 mol���ʵ�D��Һ�еμ�E��Һ�������ɳ��������ʵ���֮�����Ϊ_________mol��
 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| �� |
| ||
| �� |
| ||||
|
| ||||
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
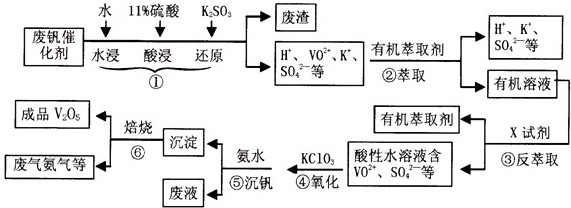
| pH | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 | 2.0 | 2.1 |
| ��������% | 88.1 | 94.8 | 96.5 | 98.0 | 98.8 | 98.8 | 96.4 | 93.1 | 89.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
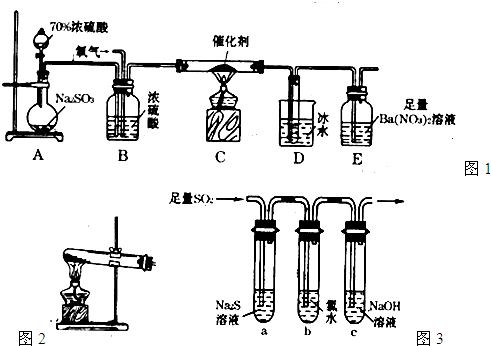
(8��)��Է���������С�������е�X��Y��Z�������嵥�ʣ���������ֵ��ʵ�Ԫ�طֱ�λ�ڲ�ͬ�Ķ����ڡ���һ�������£�X��Y��������M��X��Z��������N��M����N��������A��ʵ���ҿɷֱ�����ͼ��ʾ�ķ���װ����ȡX��Z��M(�г�װ������)��
(1)��ȡX��Z��M�ķ���װ�÷ֱ���(��д���)X��________��Z��________��M��________��
(2)X��Y��Z���ֵ��ʷֱ���X��________��Y��________��Z��________��
(3)A�ĵ���ʽ��________��A�к��еĻ�ѧ��������__________________��
(4)��֪Z����M�ڳ����·�Ӧ����Y��ͬʱ�а��̲�������Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
����ͼ��ʾװ�ý���Z��M�ڳ����·�Ӧ��ʵ�飬���ռ�Y��
������A���ݳ������庬��Z����ͨ�뷴Ӧװ��A�е�Z��M�����ʵ���֮��Ӧ����________��
������A���ݳ����������ۺ���Z��M����ϴ��ƿB���ܱ����գ���ϴ��ƿB���Լ���Z��M��Ӧ�����ӷ���ʽ�ֱ���__________________��__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�������ѧ�ڻ�ѧһ�ָ�ϰ���۽ṹ�����ʵĶ����ԡ�ר���ۺϲ��ԣ��ս̰棩 ���ͣ�ʵ����
(8��)��Է���������С�������е�X��Y��Z�������嵥�ʣ���������ֵ��ʵ�Ԫ�طֱ�λ�ڲ�ͬ�Ķ����ڡ���һ�������£�X��Y��������M��X��Z��������N��M����N��������A��ʵ���ҿɷֱ�����ͼ��ʾ�ķ���װ����ȡX��Z��M(�г�װ������)��
(1)��ȡX��Z��M�ķ���װ�÷ֱ���(��д���)X��________��Z��________��M��________��
(2)X��Y��Z���ֵ��ʷֱ���X��________��Y��________��Z��________��
(3)A�ĵ���ʽ��________��A�к��еĻ�ѧ��������__________________��
(4)��֪Z����M�ڳ����·�Ӧ����Y��ͬʱ�а��̲�������Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
����ͼ��ʾװ�ý���Z��M�ڳ����·�Ӧ��ʵ�飬���ռ�Y��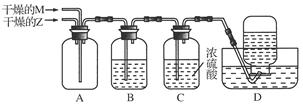
������A���ݳ������庬��Z����ͨ�뷴Ӧװ��A�е�Z��M�����ʵ���֮��Ӧ����________��
������A���ݳ����������ۺ���Z��M����ϴ��ƿB���ܱ����գ���ϴ��ƿB���Լ���Z��M��Ӧ�����ӷ���ʽ�ֱ���__________________��__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�������ѧ�ڻ�ѧһ�ָ�ϰ���۽ṹ�����ʵĶ����ԡ�ר���ۺϲ��ԣ��ս̰棩 ���ͣ�ʵ����
(8��)��Է���������С�������е�X��Y��Z�������嵥�ʣ���������ֵ��ʵ�Ԫ�طֱ�λ�ڲ�ͬ�Ķ����ڡ���һ�������£�X��Y��������M��X��Z��������N��M����N��������A��ʵ���ҿɷֱ�����ͼ��ʾ�ķ���װ����ȡX��Z��M(�г�װ������)��

(1)��ȡX��Z��M�ķ���װ�÷ֱ���(��д���)X��________��Z��________��M��________��
(2)X��Y��Z���ֵ��ʷֱ���X��________��Y��________��Z��________��
(3)A�ĵ���ʽ��________��A�к��еĻ�ѧ��������__________________��
(4)��֪Z����M�ڳ����·�Ӧ����Y��ͬʱ�а��̲�������Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
����ͼ��ʾװ�ý���Z��M�ڳ����·�Ӧ��ʵ�飬���ռ�Y��

������A���ݳ������庬��Z����ͨ�뷴Ӧװ��A�е�Z��M�����ʵ���֮��Ӧ����________��
������A���ݳ����������ۺ���Z��M����ϴ��ƿB���ܱ����գ���ϴ��ƿB���Լ���Z��M��Ӧ�����ӷ���ʽ�ֱ���__________________��__________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com